7-Keto-DHEA
7-Ketodehydroepiandrosterone (7-keto-DHEA), also known as 7-oxoprasterone, is a prohormone produced by metabolism of the prohormone dehydroepiandrosterone (DHEA).[1] 7-Keto-DHEA is not directly converted to testosterone or estrogen, and has thus been investigated as a potentially more useful relative of DHEA.[2][3] Researchers have raised concern that supplements may trigger positive tests for performance-enhancing drugs.[4][5]
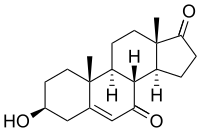 7-Keto-DHEA | |
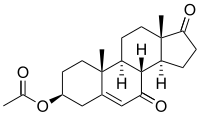 7-Keto-DHEA acetate | |
| Names | |
|---|---|
| IUPAC name
(3β)-3-Hydroxyandrost-5-ene-7,17-dione | |
| Other names
7-Oxo-DHEA; 7-Ketodehydroepiandrosterone; 7-Oxodehydroepiandrosterone; 3β-Hydroxyandrost-5-ene-7,17-dione; Androst-5-en-3β-ol-7,17-dione | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII |
|
| |
| |
| Properties | |
| C19H26O3 | |
| Molar mass | 302.414 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
The World Anti-Doping Agency lists 7-keto-DHEA as a prohibited anabolic agent.[6] The FDA has proposed that it be banned from used in compounded drugs.[7]
Chemistry
7-Keto-DHEA has a number of chemical names, including:
- 7-Ketodehydroepiandrosterone (7-keto-DHEA)
- 7-Oxodehydroepiandrosterone (7-oxo-DHEA)
- 7-Ketoprasterone
- 7-Oxoprasterone
- 3β-Hydroxyandrost-5-ene-7,17-dione
- Androst-5-en-3β-ol-7,17-dione
For the acetate ester:
- 3β-Acetoxyandrost-5-ene-7,17-dione
- 7-Oxo-dehydroepiandrosterone acetate (7-oxo-DHEA acetate)
- 3-Acetyl-7-oxo-dehydroepiandrosterone (3-acetyl-7-oxo-DHEA)
- DHEA acetate-7-one
- Δ5-Androstene-3β-acetoxy-7,17-dione
Note: "Keto" can be substituted for "oxo" in the above names.
History
7-Keto-DHEA has been marketed for treatment of the fake disease Adrenal fatigue.[7]
Regulation
The FDA has proposed that 7-Keto-DHEA be included among substances banned from use in compounded drugs.[7]
See also
References
- Worrel ME; Gurkovskaya OV; Leonard ST; Lewis PB; Winsauer PJ (June 2011). "Effects of 7-keto dehydroepiandrosterone on voluntary ethanol intake in male rats". Alcohol. 45 (4): 349–54. doi:10.1016/j.alcohol.2010.08.020. PMC 3095668. PMID 21051179.
- Lardy, H; Kneer N; Wei Y; Partridge B; Marwah P (1998). "Ergosteroids II: Biologically Active Metabolites and Synthetic Derivatives of Dehydroepiandrosterone". Steroids. 63 (3): 158–165. doi:10.1016/S0039-128X(97)00159-1. PMID 9558717.
- Davidson, M; Marwah A; Sawchuk RJ; Maki K; Marwah P; Weeks C; Lardy H (2000). "Safety and Pharmacokinetic Study with Escalating Doses of 3-acetyl-7-oxo-dehydroepiandrosterone in Healthy Male Volunteers". Clin. Invest. Med. 23 (5): 300–310. PMID 11055323.
- Delbeke FT; Van Eenoo P; Van Thuyne W; Desmet N (December 2002). "Prohormones and sport". J. Steroid Biochem. Mol. Biol. 83 (1–5): 245–51. doi:10.1016/S0960-0760(02)00274-1. PMID 12650722.
- "7-Keto DHEA". Martindale: The Complete Drug Reference. Thomson Healthcare. July 10, 2011.
7-Keto-DHEA is reported to be an anabolic androgenic steroid that may be subject to abuse in sport.
- "The World Anti-Doping Code International Standard Prohibited List January 2018" (PDF). World Anti-Doping Agency. Retrieved October 21, 2017.
- Jann Bellamy (26 September 2019). "FDA proposes ban on curcumin and other naturopathic favorites in compounded drugs". Science-Based Medicine.