Transition metal isocyanide complexes
Transition metal isocyanide complexes are coordination compounds containing isocyanide ligands. Because isocyanide are relatively basic, but also good pi-acceptors, a wide range of complexes are known. Some isocyanide complexes are used in medical imaging.
_6Cation.png)
Scope of isocyanides
9((CNCH2)3CMe%2C_VIGYUF.png)
Isocyanides are electronically similar to CO but for most R groups, isocyanides are superior Lewis bases and weaker pi-acceptors. Typical isonitrile ligands are methyl isocyanide, tert-butyl isocyanidephenyl isocyanide, and cyclohexylisocyanide. Trifluoromethylisocyanide is the exception, its coordination properties are very similarly to those of CO.
Because the CNC linkage is linear, the cone angle of these ligands is small, so it is easy to prepare polyisocyanide complexes. Many complexes of isocyanides show high coordination numbers, e.g. the eight-coordinate cation [Nb(CNBu-t)6I2]+.[3] Very bulky isocyanide ligands are also known, e.g. C6H3-2,6-Ar2-NC (Ar =aryl).[4]
Di- and triisocyanide ligands are well developed, e.g. (CH2)n(NC)2. Usually steric factors force these ligands to bind to two separate metals, i.e., they are binucleating ligands.[5] Chelating diisocyanide ligands require elaborate backbones.[6]
Synthesis and reactions
5_(PTBICF10).png)
Because of their low steric profile and high basicity, isocyanide ligands often install easily, e.g. by treating metal halides with the isocyanide. Many metal cyanides can be N-alkylated to give isocyanide complexes.[8]
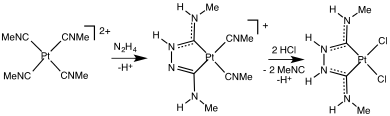
Cationic complexes are susceptible to nucleophilic attack at carbon. In this way, the first metal carbene complexes where prepared. Because isocyanides are both acceptors and donors, they stabiilize a broader range of oxidation states than does CO. This advantage is illustrated by the isolation of the homoleptic vanadium hexaisocyanide complex in three oxidation states, [V(CNC6H3-2,6-Me2)6]n for n = -1, 0, +1.[10]
Because isocyanides are more basic donors ligands than CO, their complexes are susceptible to oxidation and protonation. Thus, Fe(tBuNC)5 is easily protonated, whereas its counterpart Fe(CO)5 is not.[7]
Isocyanide has been shown to insert into metal-alkyl bonds to form iminoacyls.[11]
See also
- Cyanometalate - coordination compounds containing cyanide ligands (coordinating via C)
- Transition metal nitrile complexes - coordination compounds containing nitrile ligands, which are isomers of isonitriles
References
- Underwood, S. R.; Anagnostopoulos, C.; Cerqueira, M.; Ell, P. J.; Flint, E. J.; Harbinson, M.; Kelion, A. D.; Al-Mohammad, A.; Prvulovich, E. M.; Shaw, L. J.; Tweddel, A. C. (1 February 2004). "Myocardial perfusion scintigraphy: the evidence". European Journal of Nuclear Medicine and Molecular Imaging. 31 (2): 261–291. doi:10.1007/s00259-003-1344-5. PMC 2562441. PMID 15129710.
- Constable, Edwin C.; Johnson, Brian F.G.; Khan, Fatima K.; Lewis, Jack; Raithby, Paul R.; Mikulcik, Patrizia (1991). "Synthesis and crystal and molecular structure of Os3(CO)9[1,1,1-tris(isocyanomethyl)ethane]: A novel tris-isocyanide ligand capped triosmium cluster". Journal of Organometallic Chemistry. 403 (1–2): C15–C18. doi:10.1016/0022-328X(91)83111-G.
- Collazo, César; Rodewald, Dieter; Schmidt, Hauke; Rehder, Dieter (1996). "Niobium-Centered C−C Coupling of Isonitriles". Organometallics. 15 (22): 4884–4887. doi:10.1021/om960510v.
- Drance, Myles J.; Sears, Jeffrey D.; Mrse, Anthony M.; Moore, Curtis E.; Rheingold, Arnold L.; Neidig, Michael L.; Figueroa, Joshua S. (2019). "Terminal coordination of diatomic boron monofluoride to iron". Science. 363 (6432): 1203–1205. Bibcode:2019Sci...363.1203D. doi:10.1126/science.aaw6102. PMID 30872521. S2CID 78094683.
- Harvey, P. (2001). "Chemistry, properties and applications of the assembling 1,8-diisocyano-p-menthane, 2,5-dimethyldiisocyanohexane and 1,3-diisocyanopropane ligands and their coordination polynuclear complexes". Coordination Chemistry Reviews. 219-221: 17–52. doi:10.1016/S0010-8545(00)00415-X.
- Plummer, Daniel T.; Angelici, Robert J. (1983). "Synthesis and characterization of homoleptic complexes of the chelating bidentate isocyano ligand tert-BuDiNC". Inorganic Chemistry. 22 (26): 4063–4070. doi:10.1021/ic00168a048.
- Bassett, Jean-Maria; Farrugia, Louis J.; Stone, F. Gordon A. (1980). "Notes. Protonation of pentakis(t-butyl isocyanide)iron". Journal of the Chemical Society, Dalton Transactions (9): 1789. doi:10.1039/DT9800001789.
- Fehlhammer, Wolf P.; Fritz, Marcus. (1993). "Emergence of a CNH and cyano complex based organometallic chemistry". Chemical Reviews. 93 (3): 1243–1280. doi:10.1021/cr00019a016.
- Hahn FE, Jahnke MC (2008). "Heterocyclic carbenes: synthesis and coordination chemistry". Angewandte Chemie. 47 (17): 3122–72. doi:10.1002/anie.200703883. PMID 18398856.
- Barybin, Mikhail V.; Young, Victor G.; Ellis, John E. (2000). "First Paramagnetic Zerovalent Transition Metal Isocyanides. Syntheses, Structural Characterizations, and Magnetic Properties of Novel Low-Valent Isocyanide Complexes of Vanadium1". Journal of the American Chemical Society. 122 (19): 4678–4691. doi:10.1021/ja000212w.
- Vicente, J; Abad, J.A.; Fortsch, W.; Lopez-Saez, M.J. Reactivity of ortho-palladated phenol derivatives with unsaturated molecules. Organometallics, 2004, 23, 4414-4429. DOI 10.1021/om0496131.