Homochirality
Homochirality is a uniformity of chirality, or handedness. Objects are chiral when they cannot be superposed on their mirror images. For example, the left and right hands of a human are approximately mirror images of each other but are not their own mirror images, so they are chiral. In biology, 19 of the 20 natural amino acids are homochiral, being L-chiral (left-handed), while sugars are D-chiral (right-handed).[1] Homochirality can also refer to enantiopure substances in which all the constituents are the same enantiomer (a right-handed or left-handed version of an atom or molecule), but some sources discourage this use of the term.
It is unclear whether homochirality has a purpose; however, it appears to be a form of information storage.[2] One suggestion is that it reduces entropy barriers in the formation of large organized molecules.[3] It has been experimentally verified that amino acids form large aggregates in larger abundance from an enantiopure samples of the amino acid than from racemic (enantiomerically mixed) ones.[3]
It is not clear whether homochirality emerged before or after life, and many mechanisms for its origin have been proposed.[4] Some of these models propose three distinct steps: mirror-symmetry breaking creates a minute enantiomeric imbalance, chiral amplification builds on this imbalance, and chiral transmission is the transfer of chirality from one set of molecules to another.
In biology
Amino acids are the building blocks of peptides and enzymes while sugar-peptide chains are the backbone of RNA and DNA.[5][6] In biological organisms, amino acids appear almost exclusively in the left-handed form (L-amino acids) and sugars in the right-handed form (R-sugars).[7] Since the enzymes catalyze reactions, they enforce homochirality on a great variety of other chemicals, including hormones, toxins, fragrances and food flavors.[8]:493–494 Glycine is achiral, as are some other non-proteinogenic amino acids are either achiral (such as dimethylglycine) or of the D enantiomeric form.
Biological organisms easily discriminate between molecules with different chiralities. This can affect physiological reactions such as smell and taste. Carvone, a terpenoid found in essential oils, smells like mint in its L-form and caraway in its R-form.[8]:494 Limonene tastes like lemons when right-handed and oranges when left-handed.[9]:168
Homochirality also affects the response to drugs. Thalidomide, in its left-handed form, cures morning sickness; in its right-handed form, it causes birth defects.[9]:168 Unfortunately, even if a pure left-handed version is administered, some of it can convert to the right-handed form in the patient.[10] Many drugs are available as both a racemic mixture (equal amounts of both chiralities) and an enantiopure drug (only one chirality). Depending on the manufacturing process, enantiopure forms can be more expensive to produce than stereochemical mixtures.[9]:168
Chiral preferences can also be found at a macroscopic level. Snail shells can be right-turning or left-turning helices, but one form or the other is strongly preferred in a given species. In the edible snail Helix pomatia, only one out of 20,000 is left-helical.[11]:61–62 The coiling of plants can have a preferred chirality and even the chewing motion of cows has a 10% excess in one direction.[12]
Origins
Symmetry breaking
Known mechanisms for the production of non-racemic mixtures from racemic starting materials include: asymmetric physical laws, such as the electroweak interaction (via cosmic rays[13]); asymmetric environments, such as those caused by circularly polarized light, quartz crystals, or the Earth's rotation; and statistical fluctuations during racemic synthesis.[14] Once established, chirality would be selected for.[15] A small enantiomeric excess can be amplified into a large one by asymmetric autocatalysis, such as in the Soai reaction.[16] In asymmetric autocatalysis, the catalyst is a chiral molecule, which means that a chiral molecule is catalysing its own production. An initial enantiomeric excess, such as can be produced by polarized light, then allows the more abundant enantiomer to outcompete the other.[17]
One supposition is that the discovery of an enantiomeric imbalance in molecules in the Murchison meteorite supports an extraterrestrial origin of homochirality: there is evidence for the existence of circularly polarized light originating from Mie scattering on aligned interstellar dust particles which may trigger the formation of an enantiomeric excess within chiral material in space.[11]:123–124 Interstellar and near-stellar magnetic fields can align dust particles in this fashion.[18] Another speculation (the Vester-Ulbricht hypothesis) suggests that fundamental chirality of physical processes such as that of the beta decay (see Parity violation) leads to slightly different half-lives of biologically relevant molecules. Homochirality may also result from spontaneous absolute asymmetric synthesis.[19][20]
It is also possible that homochirality is simply a result of the natural autoamplification process of life—that either the formation of life as preferring one chirality or the other was a chance rare event which happened to occur with the chiralities we observe, or that all chiralities of life emerged rapidly but due to catastrophic events and strong competition, the other unobserved chiral preferences were wiped out by the preponderance and metabolic, enantiomeric enrichment from the 'winning' chirality choices. The emergence of chirality consensus as a natural autoamplification process has been associated with the 2nd law of thermodynamics.[21]
Amplification
Theory
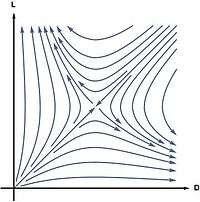
In 1953, Charles Frank proposed a model to demonstrate that homochirality is a consequence of autocatalysis.[22] In his model the L and D enantiomers of a chiral molecule are autocatalytically produced from an achiral molecule A
- A + L → 2L, A + D → 2D
while suppressing each other through a reaction that he called mutual antagonism
- L + D → ∅.
In this model the racemic state is unstable in the sense that the slightest enantiomeric excess will be amplified to a completely homochiral state. This can be shown by computing the reaction rates from the law of mass action:
where is the rate constant for the autocatalytic reactions, is the rate constant for mutual antagonism reaction, and the concentration of A is kept constant for simplicity. By defining the enantiomeric excess as
we can compute the rate of change of enatiomeric excess using chain rule from the rate of change of the concentrations of enantiomeres L and D.
Linear stability analysis of this equation shows that the racemic state is unstable. Starting from almost everywhere in the concentration space, the system evolves to a homochiral state.
It is generally understood that autocatalysis alone does not yield to homochirality, and the presence of the mutually antagonistic relationship between the two enantiomers is necessary for the instability of the racemic mixture. However, recent studies show that homochirality could be achieved from autocatalysis in the absence of the mutually antagonistic relationship, but the underlying mechanism for symmetry-breaking is different.[4][23]
Experiments
There are several laboratory experiments that demonstrate how a small amount of one enantiomer at the start of a reaction can lead to a large excess of a single enantiomer as the product. For example, the Soai reaction is autocatalytic. If the reaction is started with some of one of the product enantiomers already present, the product acts as an enantioselective catalyst for production of more of that same enantiomer.[24] The initial presence of just 0.2 equivalent one enantiomer can lead to up to 93% enantiomeric excess of the product.
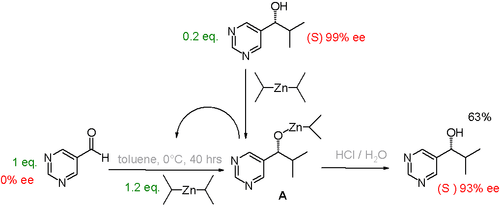
Another study[25] concerns the proline catalyzed aminoxylation of propionaldehyde by nitrosobenzene. In this system, a small enantiomeric excess of catalyst leads to a large enantiomeric excess of product.
Serine octamer clusters[26][27] are also contenders. These clusters of 8 serine molecules appear in mass spectrometry with an unusual homochiral preference, however there is no evidence that such clusters exist under non-ionizing conditions and amino acid phase behavior is far more prebiotically relevant.[28] The recent observation that partial sublimation of a 10% enantioenriched sample of leucine results in up to 82% enrichment in the sublimate shows that enantioenrichment of amino acids could occur in space.[29] Partial sublimation processes can take place on the surface of meteors where large variations in temperature exist. This finding may have consequences for the development of the Mars Organic Detector scheduled for launch in 2013 which aims to recover trace amounts of amino acids from the Mars surface exactly by a sublimation technique.
A high asymmetric amplification of the enantiomeric excess of sugars are also present in the amino acid catalyzed asymmetric formation of carbohydrates[30]
One classic study involves an experiment that takes place in the laboratory.[31] When sodium chlorate is allowed to crystallize from water and the collected crystals examined in a polarimeter, each crystal turns out to be chiral and either the L form or the D form. In an ordinary experiment the amount of L crystals collected equals the amount of D crystals (corrected for statistical effects). However, when the sodium chlorate solution is stirred during the crystallization process the crystals are either exclusively L or exclusively D. In 32 consecutive crystallization experiments 14 experiments deliver D-crystals and 18 others L-crystals. The explanation for this symmetry breaking is unclear but is related to autocatalysis taking place in the nucleation process.
In a related experiment, a crystal suspension of a racemic amino acid derivative continuously stirred, results in a 100% crystal phase of one of the enantiomers because the enantiomeric pair is able to equilibrate in solution (compare with dynamic kinetic resolution).[32]
Transmission
Many strategies in asymmetric synthesis are built on chiral transmission. Especially important is the so-called organocatalysis of organic reactions by proline for example in Mannich reactions.
Optical resolution in racemic amino acids
There exists no theory elucidating correlations among L-amino acids. If one takes, for example, alanine, which has a small methyl group, and phenylalanine, which has a larger benzyl group, a simple question is in what aspect, L-alanine resembles L-phenylalanine more than D-phenylalanine, and what kind of mechanism causes the selection of all L-amino acids. Because it might be possible that alanine was L and phenylalanine was D.
It was reported[33] in 2004 that excess racemic D,L-asparagine (Asn), which spontaneously forms crystals of either isomer during recrystallization, induces asymmetric resolution of a co-existing racemic amino acid such as arginine (Arg), aspartic acid (Asp), glutamine (Gln), histidine (His), leucine (Leu), methionine (Met), phenylalanine (Phe), serine (Ser), valine (Val), tyrosine (Tyr), and tryptophan (Trp). The enantiomeric excess ee = 100 ×(L-D)/(L+D) of these amino acids was correlated almost linearly with that of the inducer, i.e., Asn. When recrystallizations from a mixture of 12 D,L-amino acids (Ala, Asp, Arg, Glu, Gln, His, Leu, Met, Ser, Val, Phe, and Tyr) and excess D,L-Asn were made, all amino acids with the same configuration with Asn were preferentially co-crystallized.[33] It was incidental whether the enrichment took place in L- or D-Asn, however, once the selection was made, the co-existing amino acid with the same configuration at the α-carbon was preferentially involved because of thermodynamic stability in the crystal formation. The maximal ee was reported to be 100%. Based on these results, it is proposed that a mixture of racemic amino acids causes spontaneous and effective optical resolution, even if asymmetric synthesis of a single amino acid does not occur without an aid of an optically active molecule.
This is the first study elucidating reasonably the formation of chirality from racemic amino acids with experimental evidences.
History of term
This term was introduced by Kelvin in 1904, the year that he published his Baltimore Lecture of 1884. Kelvin used the term homochirality as a relationship between two molecules, i.e. two molecules are homochiral if they have the same chirality.[30][34] Recently, however, homochiral has been used in the same sense as enantiomerically pure. This is permitted in some journals (but not encouraged),[35]:342[36] its meaning changing into the preference of a process or system for a single optical isomer in a pair of isomers in these journals.
See also
- Chiral life concept - of artificially synthesizing chiral-mirror version of life
- CIP system
- Stereochemistry
- Pfeiffer Effect
- Unsolved problems in chemistry
References
- Nelson, Lehninger; et al. (2008). Lehninger Principles of Biochemistry. Macmillan. p. 474.
- Carroll, James D. (March 2009). "A new definition of life". Chirality. 21 (3): 354–358. doi:10.1002/chir.20590. PMID 18571800.
- Julian, Ryan R.; Myung, Sunnie; Clemmer, David E. (January 2005). "Do Homochiral Aggregates Have an Entropic Advantage?". The Journal of Physical Chemistry B. 109 (1): 440–444. doi:10.1021/jp046478x. PMID 16851034.
- Jafarpour, Farshid; Biancalani, Tommaso; Goldenfeld, Nigel (2017). "Noise-induced symmetry breaking far from equilibrium and the emergence of biological homochirality". Physical Review E. 95 (3): 032407. Bibcode:2017PhRvE..95c2407J. doi:10.1103/PhysRevE.95.032407. PMID 28415353.
- Reusch, William. "Peptides & Proteins". Natural Products. Michigan State University. Retrieved 8 May 2018.
- Lam, Eric (1997). "Nucleic acids and proteins". In Dey, P.M.; Harborne, J.B. (eds.). Plant Biochemistry. Burlington: Elsevier. p. 315. ISBN 9780080525723.
- Zubay, Geoffrey (2000). Origins of Life: On Earth and in the Cosmos. Elsevier. p. 96. ISBN 9780080497617.
- Seckbach, edited by Joseph (2012). Genesis - in the beginning : precursors of life, chemical models and early biological evolution. Dordrecht: Springer. ISBN 9789400729407.CS1 maint: extra text: authors list (link)
- Hazen, Robert M. (2007). Genesis : the scientific quest for life's origins. Washington, D.C.: Joseph Henry. ISBN 9780309103107.
- Smith, Silas (July 2009). "Chiral Toxicology: It's the Same Thing... Only Different". Toxicological Sciences. 110 (1): 4–30. doi:10.1093/toxsci/kfp097. PMID 19414517. Retrieved 16 April 2016.
- Meierhenrich, Uwe (2008). Amino acids and the asymmetry of life caught in the act of formation. Berlin: Springer. ISBN 9783540768869.
- Shaw, Andrew M. (2007). Astrochemistry From Astronomy to Astrobiology. Chichester: John Wiley & Sons. p. 247. ISBN 9780470091388.
- Globus, Noemie; Blandford, Roger D. (20 May 2020). "The Chiral Puzzle of Life". The Astrophysical Journal Letters. 895 (1). doi:10.3847/2041-8213/ab8dc6. Retrieved 3 July 2020.
- Plasson, Raphaël; Kondepudi, Dilip K.; Bersini, Hugues; et al. (August 2007). "Emergence of homochirality in far-from-equilibrium systems: Mechanisms and role in prebiotic chemistry". Chirality. 19 (8): 589–600. doi:10.1002/chir.20440. ISSN 0899-0042. PMID 17559107. "Special Issue: Proceedings from the Eighteenth International Symposium on Chirality (ISCD-18), Busan, Korea, 2006"
- Clark, Stuart (July–August 1999). "Polarized Starlight and the Handedness of Life". American Scientist. 87 (4): 336. Bibcode:1999AmSci..87..336C. doi:10.1511/1999.4.336. ISSN 0003-0996.
- Shibata, Takanori; Morioka, Hiroshi; Hayase, Tadakatsu; et al. (17 January 1996). "Highly Enantioselective Catalytic Asymmetric Automultiplication of Chiral Pyrimidyl Alcohol". Journal of the American Chemical Society. 118 (2): 471–472. doi:10.1021/ja953066g. ISSN 0002-7863.
- Soai, Kenso; Sato, Itaru; Shibata, Takanori (2001). "Asymmetric autocatalysis and the origin of chiral homogeneity in organic compounds". The Chemical Record. 1 (4): 321–332. doi:10.1002/tcr.1017. ISSN 1528-0691. PMID 11893072.
- Helman, Daniel S (6 July 2018). "Galactic Distribution of Chirality Sources of Organic Molecules". Acta Astronautica. 151: 595–602. arXiv:1612.06720. Bibcode:2018AcAau.151..595H. doi:10.1016/j.actaastro.2018.07.008. ISSN 0094-5765.
- Rajan, Aruna. "How did protein amino acids get left-handed while sugars got right-handed?" (PDF). Term Paper for Physics 569*. Retrieved June 18, 2014.
- "Interview: In the beginning..." Highlights in Chemical Science (5). 2008. Retrieved June 18, 2014.
- Jaakkola, S., Sharma, V. and Annila, A. (2008). "Cause of chirality consensus". Curr. Chem. Biol. 2 (2): 53–58. arXiv:0906.0254. doi:10.2174/187231308784220536.CS1 maint: multiple names: authors list (link)
- Frank, F.C. (1953). "On spontaneous asymmetric synthesis". Biochimica et Biophysica Acta. 11 (4): 459–463. doi:10.1016/0006-3002(53)90082-1. PMID 13105666.
- Jafarpour, Farshid; Biancalani, Tommaso; Goldenfeld, Nigel (2015). "Noise-induced mechanism for biological homochirality of early life self-replicators". Physical Review Letters. 115 (15): 158101. arXiv:1507.00044. Bibcode:2015PhRvL.115o8101J. doi:10.1103/PhysRevLett.115.158101. PMID 26550754.
- Takanori Shibata; Hiroshi Morioka; Tadakatsu Hayase; Kaori Choji; Kenso Soai (1996). "Highly Enantioselective Catalytic Asymmetric Automultiplication of Chiral Pyrimidyl Alcohol". J. Am. Chem. Soc. 118 (2): 471–472. doi:10.1021/ja953066g.
- Suju P. Mathew, Hiroshi Iwamura and Donna G. Blackmond (21 June 2004). "Amplification of Enantiomeric Excess in a Proline-Mediated Reaction". Angewandte Chemie International Edition. 43 (25): 3317–3321. doi:10.1002/anie.200453997. PMID 15213963.
- Cooks, R. G., Zhang, D., Koch, K. J. (2001). "Chiroselective Self-Directed Octamerization of Serine: Implications for Homochirogenesis". Anal. Chem. 73 (15): 3646–3655. doi:10.1021/ac010284l. PMID 11510829.CS1 maint: multiple names: authors list (link)
- Nanita, S., Cooks, R. G. (2006). "Serine Octamers: Cluster Formation, Reactions, and Implications for Biomolecule Homochirality". Angew. Chem. Int. Ed. 45 (4): 554–569. doi:10.1002/anie.200501328. PMID 16404754.CS1 maint: multiple names: authors list (link)
- Donna G. Blackmond; Martin Klussmann (2007). "Spoilt for choice: assessing phase behaviour models for the evolution of homochirality". Chem. Commun. (39): 3990–3996. doi:10.1039/b709314b. PMID 17912393.
- Stephen P. Fletcher; Richard B. C. Jagt; Ben L. Feringa (2007). "An astrophysically relevant mechanism for amino acid enantiomer enrichment". Chem. Commun. 2007 (25): 2578–2580. doi:10.1039/b702882b. PMID 17579743.
- Armando Córdova; Magnus Engqvist; Ismail Ibrahem; Jesús Casas; Henrik Sundén (2005). "Plausible origins of homochirality in the amino acid catalyzed neogenesis of carbohydrates". Chem. Commun. 15 (15): 2047–2049. doi:10.1039/b500589b. PMID 15834501.
- Kondepudi, D. K., Kaufman, R. J. & Singh, N. (1990). "Chiral Symmetry Breaking in Sodium Chlorate Crystallization". Science. 250 (4983): 975–976. Bibcode:1990Sci...250..975K. doi:10.1126/science.250.4983.975. PMID 17746924.CS1 maint: multiple names: authors list (link)
- Noorduin, Wim L.; Izumi, Toshiko; Millemaggi, Alessia; Leeman, Michel; Meekes, Hugo; Van Enckevort, Willem J. P.; Kellogg, Richard M.; Kaptein, Bernard; Vlieg, Elias; Blackmond, Donna G. (January 2008). "Emergence of a Single Solid Chiral State from a Nearly Racemic Amino Acid Derivative" (PDF). Journal of the American Chemical Society. 130 (4): 1158–1159. doi:10.1021/ja7106349. PMID 18173274.
- S. Kojo; H. Uchino; M. Yoshimura; K. Tanaka (2004). "Racemic D,L-asparagine causes enantiomeric excess of other coexisting racemic D,L-amino acids during recrystallization: a hypothesis accounting for the origin of L-amino acids in the biosphere". Chem. Comm. (19): 2146–2147. doi:10.1039/b409941a. PMID 15467844.
- Morris, David G. (2001). Stereochemistry. Cambridge: Royal Society of Chemistry. p. 30. ISBN 978-1-84755-194-8.
- Anslyn, Eric V.; Dougherty, Dennis A. (2006). Modern physical organic chemistry. Sausalito, Calif.: University Science Books. ISBN 9781891389313.
- However, the message can be confusing. In Moss, G. P. (1 January 1996). "Basic terminology of stereochemistry (IUPAC Recommendations 1996)" (PDF). Pure and Applied Chemistry. 68 (12): 2193–2222. doi:10.1351/pac199668122193. Retrieved 7 May 2018., the entry for Enantiomerically Pure/Enantiopure says "Use of homochiral as a synonym is strongly discouraged"; but the entry for Homochiral says "See enantiomerically pure/enantiopure."
Further reading
- Bailey, Jeremy (28 August 1998). "Stellar Circular Polarization and Biomolecular Homochirality". ScienceWeek. Archived from the original on 30 November 2010. Retrieved 5 May 2018.
- Ball, Philip (24 April 2009). "A circular argument?". Nature. doi:10.1038/news.2009.390.
- Brazil, Rachel (26 October 2015). "The origin of homochirality". Chemistry World. Retrieved 10 August 2018.
- Blackmond, Donna G.; Miller, Rom (21 June 2004). "How left-handed amino acids got ahead: a demonstration of the evolution of biological homochirality in the lab". www.imperial.ac.uk (Press release). Imperial College London. Retrieved 5 May 2018.
- Guijarro, Albert; Yus, Miguel (2008). The origin of chirality in the molecules of life : a revision from awareness to the current theories and perspectives of this unsolved problem. Cambridge, UK: Royal Society of Chemistry. ISBN 9780854041565.
- Hegstrom, Roger A.; Kondepudi, Dilip K. (1990). "The Handedness of the Universe". Scientific American. 262 (1): 108–115. Bibcode:1990SciAm.262a.108H. doi:10.1038/scientificamerican0190-108. JSTOR 24996649.
- Jafarpour, Farshid; Biancalani, Tommaso; Goldenfeld, Nigel (10 March 2017). "Noise-induced symmetry breaking far from equilibrium and the emergence of biological homochirality" (PDF). Physical Review E. 95 (3): 032407. Bibcode:2017PhRvE..95c2407J. doi:10.1103/PhysRevE.95.032407. PMID 28415353.
- Nansheng, Zhao (1999). "The role of homochirality in evolution". In Zucchi, C.; Caglioti, L.; Pályi, G. (eds.). Advances in BioChirality. Burlington: Elsevier. pp. 105–114. doi:10.1016/B978-008043404-9/50008-5. ISBN 9780080526621.
- Rouhi, A. Maureen (17 June 2004). "On the Genesis of Homochirality". Chemical & Engineering News. American Chemical Society. Retrieved 5 May 2018.
- Sedbrook, Danielle (28 July 2016). "Must the Molecules of Life Always be Left-Handed or Right-Handed?". Smithsonian. Retrieved 10 August 2018.
External links
- Observations Support Homochirality Theory. Photonics TechnologyWorld November 1998.
- Origins of Homochirality. Conference in Nordita Stockholm, February 2008.