Influenza vaccine
Influenza vaccines, also known as flu shots or flu jabs, are vaccines that protect against infection by influenza viruses.[2] New versions of the vaccines are developed twice a year, as the influenza virus rapidly changes.[2] While their effectiveness varies from year to year, most provide modest to high protection against influenza.[2][3] The United States Centers for Disease Control and Prevention (CDC) estimates that vaccination against influenza reduces sickness, medical visits, hospitalizations, and deaths.[4][5] Immunized workers who do catch the flu, return to work half a day sooner on average.[6] Vaccine effectiveness in those over 65 years old remains uncertain due to a lack of high quality research.[7][8] Vaccinating children may protect those around them.[2]
 US Navy crew member receiving an influenza vaccination | |
| Vaccine description | |
|---|---|
| Target disease | influenza virus |
| Type | inactivated, attenuated, recombinant |
| Clinical data | |
| Trade names | Afluria, Fluarix, Fluzone, others |
| AHFS/Drugs.com | Inactivated: Monograph Intranasal: Monograph Recombinant: Monograph |
| Pregnancy category | |
| Routes of administration | Intramuscular (IM), intranasal |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| ChemSpider |
|
| KEGG | |
The World Health Organization (WHO) and the US Centers for Disease Control and Prevention (CDC) recommend yearly vaccination for nearly all people over the age of six months, especially those at high risk.[2][9][10][11] The European Centre for Disease Prevention and Control (ECDC) also recommends yearly vaccination of high risk groups.[12] These groups include pregnant women, the elderly, children between six months and five years of age, those with certain health problems, and those who work in healthcare.[2][11]
The vaccines are generally safe.[2] Fever occurs in five to ten percent of children vaccinated.[2] Temporary muscle pains or feelings of tiredness may occur as well.[2] In certain years, the vaccine has been linked to an increase in Guillain–Barré syndrome among older people at a rate of about one case per million doses.[2] Although most influenza vaccines are produced using eggs, they are still recommended for people who have severe egg allergies.[13] However, influenza vaccines are not recommended in those who have had a severe allergy to previous versions of the vaccine itself.[2][13] The vaccine comes in inactive and weakened viral forms.[2] The live, weakened vaccine is generally not recommended in pregnant women, children less than two years old, adults older than 50, or people with a weakened immune system.[2] Depending on the type they can be injected into a muscle, sprayed into the nose, or injected into the middle layer of the skin (intradermal).[2] The intradermal vaccine was not available during the 2018–2019 and 2019–2020 influenza seasons.[11][14][15][16]
Vaccination against influenza began in the 1930s with large-scale availability in the United States beginning in 1945.[17][18] It is on the World Health Organization's List of Essential Medicines.[19]
Medical uses
The U.S Centers for Disease Control and Prevention (CDC) recommends the flu vaccine as the best way to protect people against the flu and prevent its spread.[20] The flu vaccine can also reduce the severity of the flu if a person contracts a strain that the vaccine did not contain.[20] It takes about two weeks following vaccination for protective antibodies to form.[20]
A 2012 meta-analysis found that flu vaccination was effective 67 percent of the time; the populations that benefited the most were HIV-positive adults aged 18 to 55 (76 percent), healthy adults aged 18 to 46 (approximately 70 percent), and healthy children aged six months to 24 months (66 percent).[21] The influenza vaccine also appear to protect against myocardial infarction with a benefit of 15 to 45%.[22]
Effectiveness
| 2004 | 10% |
|---|---|
| 2005 | 21% |
| 2006 | 52% |
| 2007 | 37% |
| 2008 | 41% |
| 2009 | 56% |
| 2010 | 60% |
| 2011 | 47% |
| 2012 | 49% |
| 2013 | 52% |
| 2014 | 19% |
| 2015 | 48% |
| 2016 | 40% |
| 2017 | 38% |
| 2018 | 29% |
| 2019 | 45% est |
A vaccine is assessed by its efficacy – the extent to which it reduces risk of disease under controlled conditions – and its effectiveness – the observed reduction in risk after the vaccine is put into use.[26] In the case of influenza, effectiveness is expected to be lower than the efficacy because it is measured using the rates of influenza-like illness, which is not always caused by influenza.[6] Influenza vaccines generally show high efficacy, as measured by the antibody production in animal models or vaccinated people.[27] However, studies on the effectiveness of flu vaccines in the real world are difficult; vaccines may be imperfectly matched, virus prevalence varies widely between years, and influenza is often confused with other influenza-like illnesses.[28] However, in most years (16 of the 19 years before 2007), the flu vaccine strains have been a good match for the circulating strains,[29] and even a mismatched vaccine can often provide cross-protection.[20] The virus rapidly changes due to antigenic drift, a slight mutation in the virus that causes a new strain to arise.[30]
Repeated annual influenza vaccination generally offer consistent year-on-year protection against influenza.[31] There is however suggestive evidence that repeated vaccinations may cause a reduction in vaccine effectiveness for certain influenza subtypes; this has no relevance to current recommendations for yearly vaccinations but might influence future vaccination policy.[32][33] As of 2019, the CDC recommends a yearly vaccine as most studies demonstrate overall effectiveness of annual influenza vaccination.[31]
Criticism
Tom Jefferson, who has led Cochrane Collaboration reviews of flu vaccines, has called clinical evidence concerning flu vaccines "rubbish" and has therefore declared them to be ineffective; he has called for placebo-controlled randomized clinical trials, which most in the field hold as unethical. His views on the efficacy of flu vaccines are rejected by medical institutions including the CDC and the National Institutes of Health, and by key figures in the field like Anthony Fauci.[34]
Michael Osterholm, who led the Center for Infectious Disease Research and Policy 2012 review on flu vaccines, recommended getting the vaccine but criticized its promotion, saying, "We have overpromoted and overhyped this vaccine...it does not protect as promoted. It's all a sales job: it's all public relations".[35]
Children
The CDC recommends that everyone except infants under the age of six months should receive the seasonal influenza vaccine.[9] Vaccination campaigns usually focus special attention on people who are at high risk of serious complications if they catch the flu, such as pregnant women, children under 59 months, the elderly, and people with chronic illnesses or weakened immune systems, as well as those to whom they are exposed, such as healthcare workers.[9][36]
As the death rate is also high among infants who catch influenza, the CDC and the WHO recommend that household contacts and caregivers of infants be vaccinated to reduce the risk of passing an influenza infection to the infant.[36][37]
In children, the vaccine appears to decrease the risk of influenza and possibly influenza-like illness.[38] In children under the age of two data is limited.[38] During the 2017–18 flu season, the CDC director indicated that 85 percent of the children who died "likely will not have been vaccinated."[39]
In the United States, as of January 2019, the CDC recommend that children aged six through 35 months may receive either 0.25 milliliters or 0.5 milliliters per dose of Fluzone Quadrivalent.[40][41] There is no preference for one or the other dose volume of Fluzone Quadrivalent for that age group.[40] All persons 36 months of age and older should receive 0.5 milliliters per dose of Fluzone Quadrivalent.[40] As of October 2018, Afluria Quadrivalent is licensed for children six months of age and older in the United States.[40][42] Children six months through 35 months of age should receive 0.25 milliliters for each dose of Afluria Quadrivalent.[40] All persons 36 months of age and older should receive 0.5 milliliters per dose of Afluria Quadrivalent.[40] As of February 2018, Afluria Tetra is licensed for adults and children five years of age and older in Canada.[43]
In 2014, the Canadian National Advisory Committee on Immunization (NACI) published a review of influenza vaccination in healthy 5–18-year-olds,[44] and in 2015, published a review of the use of pediatric Fluad in children 6–72 months of age.[45] In one study, conducted in a tertiary referral center, the rate of influenza vaccination in children was only 31%. Higher rates were found among immuno-suppressed pediatric patients (46%), and in patients with inflammatory bowel disease (50%).[46]
Adults
In unvaccinated adults, 16% get symptoms similar to the flu, while about 10% of vaccinated adults do.[6] Vaccination decreased confirmed cases of influenza from about 2.4% to 1.1%.[6] No effect on hospitalization was found.[6]
In working adults, a review by the Cochrane Collaboration found that vaccination resulted in a modest decrease in both influenza symptoms and working days lost, without affecting transmission or influenza-related complications.[6] In healthy working adults, influenza vaccines can provide moderate protection against virologically confirmed influenza, though such protection is greatly reduced or absent in some seasons.[7]
In healthcare workers, a 2006 review found a net benefit.[47] Of the eighteen studies in this review, only two also assessed the relationship of patient mortality relative to staff influenza vaccine uptake; both found that higher rates of healthcare worker vaccination correlated with reduced patient deaths.[47] A 2014 review found benefits to patients when healthcare workers were immunized, as supported by moderate evidence[48] based in part on the observed reduction in all-cause deaths in patients whose healthcare workers were given immunization compared with comparison patients where the workers were not offered vaccine.[49]
Elderly
Evidence for an effect in adults over 65 years old is unclear.[50] Systematic reviews examining both randomized controlled and case–control studies found a lack of high-quality evidence.[7][8] Reviews of case–control studies found effects against laboratory-confirmed influenza, pneumonia, and death among the community-dwelling elderly.[51][52]
The group most vulnerable to non-pandemic flu, the elderly, benefits least from the vaccine. There are multiple reasons behind this steep decline in vaccine efficacy, the most common of which are the declining immunological function and frailty associated with advanced age.[53] In a non-pandemic year, a person in the United States aged 50–64 is nearly ten times more likely to die an influenza-associated death than a younger person, and a person over age 65 is over ten times more likely to die an influenza-associated death than the 50–64 age group.[54]
There is a high-dose flu vaccine specifically formulated to provide a stronger immune response.[55] Available evidence indicates that vaccinating the elderly with the high-dose vaccine leads to a stronger immune response against influenza than the regular-dose vaccine.[56][57][58]
A flu vaccine containing an adjuvant was approved by the US Food and Drug Administration (FDA) in November 2015, for use by adults aged 65 years of age and older. The vaccine is marketed as Fluad in the US and was first available in the 2016–2017 flu season. The vaccine contains the MF59C.1 adjuvant[59] which is an oil-in-water emulsion of squalene oil. It is the first adjuvanted seasonal flu vaccine marketed in the United States.[60][61][62] It is not clear if there is a significant benefit for the elderly to use a flu vaccine containing the MF59C.1 adjuvant.[63][64][65] Per Advisory Committee on Immunization Practices guidelines, Fluad can be used as an alternative to other influenza vaccines approved for people 65 years and older.[61]
Vaccinating healthcare workers who work with elderly people is recommended in many countries, with the goal of reducing influenza outbreaks in this vulnerable population.[66][67][68] While there is no conclusive evidence from randomized clinical trials that vaccinating healthcare workers helps protect elderly people from influenza, there is tentative evidence of benefit.[69]
Fluad Quadrivalent was approved for use in the United States in February 2020[70] and Fluad Tetra was approved for use in the European Union in May 2020.[71]
Pregnancy
As well as protecting mother and child from the effects of an influenza infection, the immunization of pregnant women tends to increase their chances of experiencing a successful full-term pregnancy.[72]
The trivalent inactivated influenza vaccine is protective in pregnant women infected with HIV.[73]
Safety
While side effects of the flu vaccine may occur, they are usually minor. The flu vaccine can cause serious side effects, including an allergic reaction, but this is rare. Furthermore, the common side effects and risks are mild and temporary when compared to the risks and severe health effects of the annual influenza epidemic.[20] Flu vaccination may lead to side effects such as a runny nose and a sore throat, which can last for up to several days.
Guillain–Barré syndrome
Although Guillain–Barré syndrome had been feared as a complication of vaccination, the CDC states that most studies on modern influenza vaccines have seen no link with Guillain–Barré.[74][75] Infection with influenza virus itself increases both the risk of death (up to 1 in 10,000) and increases the risk of developing Guillain–Barré syndrome to a far higher level than the highest level of suspected vaccine involvement (approximately 10 times higher by 2009 estimates).[76][77]
Although one review gives an incidence of about one case of Guillain–Barré per million vaccinations,[78] a large study in China, covering close to 100 million doses of vaccine against the 2009 H1N1 "swine" flu found only eleven cases of Guillain–Barré syndrome, (0.1 per million doses) total incidence in persons vaccinated, actually lower than the normal rate of the disease in China, and no other notable side effects.[77][79]
Egg allergy
Although most influenza vaccines are produced using egg-based techniques, influenza vaccines are nonetheless still recommended for people with egg allergies, even if severe.[13] Studies examining the safety of influenza vaccines in people with severe egg allergies found that anaphylaxis was very rare, occurring in 1.3 cases per million doses given.[13]
Monitoring for symptoms from vaccination is recommended in those with more severe symptoms.[80] A study of nearly 800 children with egg allergy, including over 250 with previous anaphylactic reactions, had zero systemic allergic reactions when given the live attenuated flu vaccine.[81][82]
Other
Several studies have identified an increased incidence of narcolepsy among recipients of the pandemic H1N1 influenza ASO3-adjuvanted vaccine;[83] efforts to identify a mechanism for this suggest that narcolepsy is autoimmune, and that the ASO3-adjuvanted H1N1 vaccine may mimic hypocretin, serving as a trigger.[84]
Some injection-based flu vaccines intended for adults in the United States contain thiomersal (also known as thimerosal), a mercury-based preservative.[85][86] Despite some controversy in the media,[87] the World Health Organization's Global Advisory Committee on Vaccine Safety has concluded that there is no evidence of toxicity from thiomersal in vaccines and no reason on grounds of safety to change to more-expensive single-dose administration.[88]
Types
Flu vaccines are available either as:
- a trivalent or quadrivalent intramuscular injection (IIV3, IIV4, or RIV4, that is, TIV or QIV), which contains the inactivated form of the virus
- a nasal spray of live attenuated influenza vaccine (LAIV, Q/LAIV), which contains the live but attenuated (weakened) form of the virus.
TIV or QIV induce protection after injection (typically intramuscular, though subcutaneous and intradermal routes can also be protective)[89] based on an immune response to the antigens present on the inactivated virus, while cold-adapted LAIV works by establishing infection in the nasal passages.[90]
Recommendations
Various public health organizations, including the World Health Organization (WHO), recommend that yearly influenza vaccination be routinely offered, particularly to people at risk of complications of influenza and those individuals who live with or care for high-risk individuals, including:
- the elderly (UK recommendation is those aged 65 or above)
- people with chronic lung diseases (asthma, COPD, etc.)
- people with chronic heart diseases (congenital heart disease, chronic heart failure, ischaemic heart disease)
- people with chronic liver diseases (including cirrhosis)
- people with chronic kidney diseases (such as the nephrotic syndrome)
- people who have had their spleen removed or whose spleen is not working properly
- people who are immunosuppressed (people with HIV, those receiving medications to suppress the immune system, and people on chemotherapy) and their household contacts
- people who live together in large numbers in an environment where influenza can spread rapidly, such as prisons, nursing homes, schools, and dormitories.[91]
- healthcare workers (both to prevent sickness and to prevent spread to patients)[92][93]
- pregnant women. However, a 2009 review concluded that there was insufficient evidence to recommend routine use of trivalent influenza vaccine during the first trimester of pregnancy.[94] Influenza vaccination during flu season is part of recommendations for influenza vaccination of pregnant women in the United States.[95]
The flu vaccine is contraindicated for those under six months of age and those with severe, life-threatening allergies to flu vaccine or any ingredient in the vaccine.[9][96][13]
World Health Organization
As of 2016, the World Health Organization (WHO) recommends seasonal influenza vaccination for:[97][98][99][100][101]
First priority:
- Pregnant women
Second priority (in no particular order):
- Children aged 6–59 months
- Elderly
- Individuals with specific chronic medical conditions
- Health-care workers
Canada
The National Advisory Committee on Immunization (NACI), the group that advises the Public Health Agency of Canada, recommends that everyone over six months of age be encouraged to receive annual influenza vaccination, and that children between the age of six months and 24 months, and their household contacts, should be considered a high priority for the flu vaccine.[102] Particularly:
- People at high risk of influenza-related complications or hospitalization, including the morbidly obese, healthy pregnant women, children 6 to 59 months, the elderly, aboriginals, and people suffering from one of an itemized list of chronic health conditions
- People capable of transmitting influenza to those at high risk, including household contacts and healthcare workers
- People who provide essential community services
- Certain poultry workers
Live attenuated influenza vaccine (LAIV) was not available in Canada for the 2019–2020 season.[102]
European Union
The European Centre for Disease Prevention and Control (ECDC) recommends vaccinating the elderly as a priority, with a secondary priority people with chronic medical conditions and healthcare workers.[103]
The influenza vaccination strategy is generally that of protecting vulnerable people, rather than limiting influenza circulation or totally eliminating human influenza sickness. This is in contrast with the high herd immunity strategies for other infectious diseases such as polio and measles.[104] This is also due in part to the financial and logistics burden associated with the need of an annual injection.[105]
United States
In the United States routine influenza vaccination is recommended for all persons aged 6 months and over.[106][107][108] It takes up to two weeks after vaccination for sufficient antibodies to develop in the body.[108] The CDC recommends vaccination before the end of October, although it considers getting a vaccine in January or even later to be still beneficial.[20][108]
According to the CDC, the live attenuated virus (LAIV4) (which comes in the form of the nasal spray in the US) should be avoided by:[11][109]
- Children younger than two years
- Adults 50 years and older
- Concomitant aspirin- or salicylate-containing therapy in children and adolescents
- Children aged two through four years who have received a diagnosis of asthma or whose parents or caregivers report that a healthcare provider has told them during the past twelve months that their child had wheezing or asthma or whose medical record indicates that a wheezing episode has occurred within the past twelve months
- Persons who are immunocompromised due to any cause (including but not limited to medications and HIV infection)
- Close contacts and caregivers of severely immunocompromised persons who require a protected environment
- Pregnant women
- Persons who have received influenza antiviral medications within the previous 48 hours
Within its blanket recommendation for general vaccination in the United States, the CDC, which began recommending the influenza vaccine to healthcare workers in 1981, emphasizes to clinicians the special urgency of vaccination for members of certain vulnerable groups, and their caregivers:
- Vaccination is especially important for people at higher risk of serious influenza complications or people who live with or care for people at higher risk for serious complications.[110] In 2009, a new high-dose formulation of the standard influenza vaccine was approved.[111] The Fluzone High Dose is specifically for people 65 and older; the difference is that it has four times the antigen dose of the standard Fluzone.[112][113][114][115]
The US government requires hospitals to report worker vaccination rates. Some US states and hundreds of US hospitals require healthcare workers to either get vaccinations or wear masks during flu season. These requirements occasionally engender union lawsuits on narrow collective bargaining grounds, but proponents note that courts have generally endorsed forced vaccination laws affecting the general population during disease outbreaks.[116]
Vaccination against influenza is especially considered important for members of high-risk groups who would be likely to have complications from influenza, for example pregnant women[106][117] and children and teenagers from six months to 18 years of age who are receiving aspirin- or salicylate-containing medications and who might be at risk for experiencing Reye syndrome after influenza virus infection;[11][91]
- In raising the upper age limit to 18 years, the aim is to reduce both the time children and parents lose from visits to pediatricians and missing school and the need for antibiotics for complications[118]
- An added benefit expected from the vaccination of children is a reduction in the number of influenza cases among parents and other household members, and of possible spread to the general community.[118]
The CDC indicated that live attenuated influenza vaccine (LAIV), also called the nasal spray vaccine, was not recommended for the 2016–2017 flu season in the United States.[119]
Furthermore, the CDC recommends that healthcare personnel who care for severely immunocompromised persons receive injections (TIV or QIV) rather than LAIV.[120]
Australia
The Australian Government recommends seasonal flu vaccination for everyone over the age of six months.[121] Australia uses inactivated vaccines.[121] The flu vaccine is free for the following people:[122]
- children aged six months to five years
- people aged 65 years and over
- Aboriginal and Torres Strait Islander people aged six months and over
- pregnant women
- anyone over six months of age with medical conditions such as severe asthma, lung disease or heart disease, low immunity or diabetes that can lead to complications from influenza.
Uptake
| Country | Region | % aged 65+ |
|---|---|---|
| Asia | 83 | |
| Oceania | 75 | |
| Europe | 73 | |
| Americas | 68 | |
| Oceania | 65 | |
| Americas | 65 | |
| Europe | 64 | |
| Americas | 61 | |
| Europe | 61 | |
| Asia | 58 | |
| Europe | 58 | |
| Europe | 54 | |
| Europe | 53 | |
| Europe | 52 | |
| Asia | 50 | |
| Europe | 50 | |
| Europe | 49 | |
| Europe | 48 | |
| Europe | 45 | |
| Europe | 38 | |
| Europe | 35 | |
| Europe | 34 | |
| Europe | 27 | |
| Europe | 20 | |
| Europe | 13 | |
| Europe | 13 | |
| Europe | 12 | |
| Europe | 8 | |
| Asia | 7 | |
| Europe | 5 | |
At risk groups
Uptake of flu vaccination, both seasonally and during pandemics, is often low.[124] Systematic reviews of pandemic flu vaccination uptake have identified several personal factors that may influence uptake, including gender (higher uptake in men), ethnicity (higher in people from ethnic minorities) and having a chronic illness.[125][126] Beliefs in the safety and effectiveness of the vaccine are also important.[124]
A number of measures have found to be useful to increase rates of vaccination in those over 60 years old including: patient reminders using leaflets and letters, postcard reminders, client outreach programs, vaccine home visits, group vaccinations, free vaccinations, physician payment, physician reminders and encouraging physician competition.[127]
Healthcare workers
Frontline healthcare workers are often recommended to get seasonal and any pandemic flu vaccination. For example, in the UK all healthcare workers involved in patient care are recommended to receive the seasonal flu vaccine, and were also recommended to be vaccinated against the H1N1/09 (later renamed A(H1N1)pdm09[note 1][128]) swine flu virus during the 2009 pandemic. However, uptake is often low.[93] During the 2009 pandemic, low uptake by healthcare workers was seen in countries including the UK,[93] Italy,[129] Greece,[130] and Hong Kong.[131]
In a 2010 survey of United States healthcare workers, 63.5% reported that they received the flu vaccine during the 2010–11 season, an increase from 61.9% reported the previous season. US Health professionals with direct patient contact had higher vaccination uptake, such as physicians and dentists (84.2%) and nurse practitioners (82.6%).[132][133][134]
The main reason to vaccinate healthcare workers is to prevent staff from spreading flu to their patients and to reduce staff absence at a time of high service demand, but the reasons healthcare workers state for their decisions to accept or decline vaccination may more often be to do with perceived personal benefits.[93]
In Victoria (Australia) public hospitals, rates of healthcare worker vaccination in 2005 ranged from 34% for non-clinical staff to 42% for laboratory staff. One of the reasons for rejecting vaccines was concern over adverse reactions; in one study, 31% of resident physicians at a teaching hospital incorrectly believed Australian vaccines could cause influenza.[135]
Manufacturing
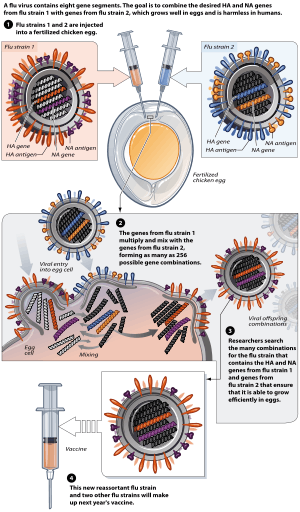
Flu vaccine is usually grown by vaccine manufacturers in fertilized chicken eggs.[136][137] In the Northern hemisphere, the manufacturing process begins following the announcement (typically in February) of the WHO recommended strains for the winter flu season.[136][138] Three strains (representing an H1N1, an H3N2, and a B strain) of flu are selected and chicken eggs are inoculated separately. These monovalent harvests are then combined to make the trivalent vaccine.[139]
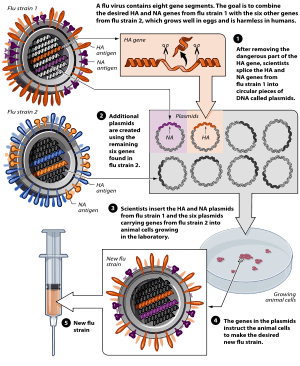
As of November 2007, both the conventional injection and the nasal spray are manufactured using chicken eggs.[137] The European Union also approved Optaflu, a vaccine produced by Novartis using vats of animal cells.[137] This technique is expected to be more scalable and avoid problems with eggs, such as allergic reactions and incompatibility with strains that affect avians like chickens.[137] Research continues into the idea of a "universal" influenza vaccine that would not require tailoring to a particular strain, but would be effective against a broad variety of influenza viruses.[140] However, no vaccine candidates had been announced by November 2007.[137]
A DNA-based vaccination, which is hoped to be even faster to manufacture, is, as of 2011, in clinical trials, determining safety and efficacy.[141][142][143]
On November 20, 2012, Novartis received FDA approval for the first cell-culture vaccine.[144][145][146][147][145]
In a 2007 report, the global capacity of approximately 826 million seasonal influenza vaccine doses (inactivated and live) was double the production of 413 million doses. In an aggressive scenario of producing pandemic influenza vaccines by 2013, only 2.8 billion courses could be produced in a six-month time frame. If all high- and upper-middle-income countries sought vaccines for their entire populations in a pandemic, nearly 2 billion courses would be required. If China pursued this goal as well, more than 3 billion courses would be required to serve these populations.[148] Vaccine research and development is ongoing to identify novel vaccine approaches that could produce much greater quantities of vaccine at a price that is affordable to the global population.
Methods of vaccine generation that bypass the need for eggs include the construction of influenza virus-like particles (VLP). VLP resemble viruses, but there is no need for inactivation, as they do not include viral coding elements, but merely present antigens in a similar manner to a virion. Some methods of producing VLP include cultures of Spodoptera frugiperda Sf9 insect cells and plant-based vaccine production (e.g., production in Nicotiana benthamiana). There is evidence that some VLPs elicit antibodies that recognize a broader panel of antigenically distinct viral isolates compared to other vaccines in the hemagglutination-inhibition assay (HIA).[149]
Influenza vaccines are produced in pathogen-free eggs that are 11 to 12 days old.[150] The top of the egg is disinfected by wiping it with alcohol and then the egg is candled to identify a non-veinous area in the allantoic cavity where a small hole is poked to serve as a pressure release.[151] A second hole is made at the top of the egg, where the influenza virus is injected in the allantoic cavity, past the chorioallantoic membrane. The two holes are then sealed with melted paraffin and the inoculated eggs are incubated for 48 hours at 37 degrees Celsius.[150] During incubation time, the virus replicates and newly replicated viruses are released into the allantoic fluid [152]
After the 48-hour incubation period, the top of the egg is cracked and the 10 milliliters of allantoic fluid is removed, from which about 15 micrograms of the flu vaccine can be obtained. At this point, the viruses have been weakened or killed and the viral antigen is purified and placed inside vials, syringes, or nasal sprayers.[152] Done on a large-scale, this method is used to produce the flu vaccine for the human population.
In 2013, the recombinant influenza vaccine, Flublok, was approved for use in the United States.[153][154][155][156]
Annual reformulation
Each year, three strains are chosen for selection in that year's flu vaccination by the WHO Global Influenza Surveillance and Response System.[157] The chosen strains are the H1N1, H3N2, and Type-B strains thought most likely to cause significant human suffering in the coming season. Starting with the 2012–2013 Northern Hemisphere influenza season (coincident with the approval of quadrivalent influenza vaccines), the WHO has also recommended a 2nd B-strain for use in quadrivalent vaccines. The World Health Organization (WHO) coordinates the contents of the vaccine each year to contain the most likely strains of the virus to attack the next year.
- "The WHO Global Influenza Surveillance Network was established in 1952 [renamed "Global Influenza Surveillance and Response System" in 2011].[158] The network comprises four WHO Collaborating Centres (WHO CCs) and 112 institutions in 83 countries, which are recognized by WHO as WHO National Influenza Centres (NICs). These NICs collect specimens in their country, perform primary virus isolation and preliminary antigenic characterization. They ship newly isolated strains to WHO CCs for high level antigenic and genetic analysis, the result of which forms the basis for WHO recommendations on the composition of influenza vaccine for the Northern and Southern Hemisphere each year."[159]
The Global Influenza Surveillance and Response System's selection of viruses for the vaccine manufacturing process is based on its best estimate of which strains will predominate the next year, amounting in the end to well-informed but fallible guesswork.[160]
Formal WHO recommendations were first issued in 1973. Beginning in 1999 there have been two recommendations per year: one for the northern hemisphere and the other for the southern hemisphere.[161]
Historical annual reformulations of the influenza vaccine are listed in a separate article. Recent WHO seasonal influenza vaccine composition recommendations:
2017–2018 Northern Hemisphere influenza season
The composition of trivalent virus vaccines for use in the 2017–2018 Northern Hemisphere influenza season recommended by the Advisory Committee on Immunization Practices on August 25, 2017[162] was:
- an A/Michigan/45/2015 (H1N1)pdm09–like virus[note 1][163]
- an A/Hong Kong/4801/2014 (H3N2)-like virus
- a B/Brisbane/60/2008–like virus (Victoria lineage)
In addition to these components, quadrivalent vaccines will also include a B/Phuket/3073/2013–like virus (Yamagata lineage).
In California, some emergency systems were strained by a spike in H3N2 flu cases. In addition, some areas experienced local shortages of oseltamivir.[164] The severity of the flu season seemed somewhat comparable to the 2009–10 swine flu outbreak. A February 2018 CDC interim report estimated the vaccine effectiveness to be 25% against H3N2, 67% against H1N1, and 42% against influenza B.[165][166]
2018 Southern Hemisphere influenza season
The composition of virus vaccines for use in the 2018 Southern Hemisphere influenza season recommended by the World Health Organization on September 28, 2017 was:[167]
- an A/Michigan/45/2015 (H1N1)pdm09-like virus[note 1][163]
- an A/Singapore/INFIMH-16-0019/2016 (H3N2)-like virus
- a B/Phuket/3073/2013-like virus
The WHO recommended that quadrivalent vaccines containing two influenza B viruses should contain the above three viruses and a B/Brisbane/60/2008-like virus.[168][167]
2018–2019 Northern Hemisphere influenza season
The composition of virus vaccines for use in the 2018–2019 Northern Hemisphere influenza season recommended by the World Health Organization on February 22, 2018 was:[169][170]
- an A/Michigan/45/2015 (H1N1)pdm09-like virus
- an A/Singapore/INFIMH-16-0019/2016 (H3N2)-like virus
- a B/Colorado/06/2017-like virus (B/Victoria/2/87 lineage)
- a B/Phuket/3073/2013-like virus (B/Yamagata/16/88 lineage)
The WHO recommended that trivalent vaccines use as their influenza B virus a B/Colorado/06/2017-like virus of the B/Victoria/2/87-lineage.[171][169] A February 2019, CDC interim report estimated the vaccine effectiveness to be approximately 47% against the 2018–2019 flu strains.[24]
The composition of virus vaccines for use in the United States for the 2018–2019 Northern Hemisphere influenza season recommended by the Food and Drug Administration on March 1, 2018, was:[172]
- an A/Michigan/45/2015 (H1N1) pdm09-like virus
- an A/Singapore/INFIMH-16–0019/2016 (H3N2)-like virus
- a B/Colorado/06/2017-like virus (B/Victoria lineage)
The committee also recommended that quadrivalent influenza vaccines contain the above three strains and the following additional B strain:[172]
- a B/Phuket/3073/2013-like virus (B/Yamagata lineage)
2019 Southern Hemisphere influenza season
The composition of virus vaccines for use in the 2019 Southern Hemisphere influenza season recommended by the World Health Organization in September 2018 was:[173]
- an A/Michigan/45/2015 (H1N1)pdm09-like virus
- an A/Switzerland/8060/2017 (H3N2)-like virus
- a B/Colorado/06/2017-like virus (B/Victoria/2/87 lineage)
- a B/Phuket/3073/2013-like virus (B/Yamagata/16/88 lineage)
The WHO recommended that trivalent vaccines use as their influenza B virus a B/Colorado/06/2017-like virus of the B/Victoria/2/87-lineage.[174]
2019–2020 Northern Hemisphere influenza season
The composition of virus vaccines for use in the 2019–2020 Northern Hemisphere influenza season recommended by the World Health Organization on March 21, 2019 was:
- an A/Brisbane/02/2018 (H1N1)pdm09-like virus[note 1][175]
- an A/Kansas/14/2017 (H3N2)-like virus
- a B/Colorado/06/2017-like virus (B/Victoria/2/87 lineage)
- a B/Phuket/3073/2013-like virus (B/Yamagata/16/88 lineage)
The WHO recommended that trivalent vaccines use as their influenza B virus a B/Colorado/06/2017-like virus of the B/Victoria/2/87-lineage.[176]
United States
2019–2020
The composition of virus vaccines for use in the United States for the 2019–2020 Northern Hemisphere influenza season recommended by the Food and Drug Administration (FDA) on March 22, 2019, was:[177][178][179]
- an A/Brisbane/02/2018 (H1N1)pdm09-like virus
- an A/Kansas/14/2017 (H3N2)-like virus
- a B/Colorado/06/2017-like virus (B/Victoria lineage)
The committee also recommended that quadrivalent influenza vaccines contain the above three strains and the following additional B strain:[177]
- a B/Phuket/3073/2013-like virus (B/Yamagata lineage)
2020–2021
The Vaccines and Related Biological Products Advisory Committee (VRBPAC) of the Food and Drug Administration (FDA) recommended that the quadrivalent formulation of egg-based influenza vaccines for the US 2020–2021 influenza season contain the following:[180]
- an A/Guangdong-Maonan/SWL1536/2019 (H1N1) pdm09-like virus;
- an A/HongKong/2671/2019 (H3N2)-like virus;
- a B/Washington/02/2019- like virus (B/Victoria lineage);
- a B/Phuket/3073/2013-like virus (B/Yamagata lineage).
The committee recommended that the quadrivalent formulation of cell- or recombinant-based influenza vaccines for the US 2020–2021 influenza season contain the following:[180]
- an A/Hawaii/70/2019 (H1N1) pdm09-like virus;
- an A/HongKong/45/2019 (H3N2)-like virus;
- a B/Washington/02/2019- like virus (B/Victoria lineage);
- a B/Phuket/3073/2013-like virus (B/Yamagata lineage)
For trivalent influenza vaccines for use in the US for the 2020–2021 influenza season, depending on the manufacturing method of the vaccine, the committee recommended that the A(H1N1) pdm09, A(H3N2) and B/Victoria lineage viruses recommended above for the quadrivalent vaccines be used.[180]
European Union
The composition of virus vaccines for use in the European Union for the 2019–2020 Northern Hemisphere influenza season recommended by the European Medicines Agency on May 15, 2019, was:[181]
Trivalent vaccines should contain:
- an A/Brisbane/02/2018 (H1N1)pdm09-like virus;
- an A/Kansas/14/2017 (H3N2)-like virus;
- a B/Colorado/06/2017-like virus (B/Victoria/2/87 lineage).
For vaccine manufacturers considering the use of a B/Yamagata/16/88 virus lineage vaccine virus in quadrivalent vaccines containing two influenza B viruses, a B/Phuket/3073/2013-like virus in addition to the strains mentioned above is considered appropriate.[181]
2020 Southern Hemisphere influenza season
The composition of virus vaccines for use in the 2020 Southern Hemisphere influenza season influenza season recommended by the World Health Organization in September 2019 was:[182]
- an A/Brisbane/02/2018 (H1N1)pdm09-like virus
- an A/South Australia/34/2019 (H3N2)-like virus
- a B/Washington/02/2019-like (B/Victoria lineage) virus
- a B/Phuket/3073/2013-like (B/Yamagata lineage) virus
The WHO recommended that trivalent vaccines use as their influenza B virus a B/Washington/02/2019-like (B/Victoria lineage) virus.[182]
History
Vaccines are used in both humans and nonhumans. Human vaccine is meant unless specifically identified as a veterinary, poultry or livestock vaccine.
Origins and development
In the worldwide Spanish flu pandemic of 1918, "Physicians tried everything they knew, everything they had ever heard of, from the ancient art of bleeding patients, to administering oxygen, to developing new vaccines and serums (chiefly against what we now call Hemophilus influenzae – a name derived from the fact that it was originally considered the etiological agent – and several types of pneumococci). Only one therapeutic measure, transfusing blood from recovered patients to new victims, showed any hint of success."[183]
In 1931, viral growth in embryonated hens' eggs was reported by Ernest William Goodpasture and colleagues at Vanderbilt University. The work was extended to growth of influenza virus by several workers, including Thomas Francis, Jonas Salk, Wilson Smith and Macfarlane Burnet, leading to the first experimental influenza vaccines.[184] In the 1940s, the US military developed the first approved inactivated vaccines for influenza, which were used in the Second World War.[185] Hen's eggs continued to be used to produce virus used in influenza vaccines, but manufacturers made improvements in the purity of the virus by developing improved processes to remove egg proteins and to reduce systemic reactivity of the vaccine.[186] In 2012, the US Food and Drug Administration (FDA) approved influenza vaccines made by growing virus in cell cultures[187][145][188] and influenza vaccines made from recombinant proteins[153] have been approved, with plant-based influenza vaccines being tested in clinical trials.[189]
Acceptance

According to the CDC: "Influenza vaccination is the primary method for preventing influenza and its severe complications. [...] Vaccination is associated with reductions in influenza-related respiratory illness and physician visits among all age groups, hospitalization and death among persons at high risk, otitis media among children, and work absenteeism among adults. Although influenza vaccination levels increased substantially during the 1990s, further improvements in vaccine coverage levels are needed".[190]
The egg-based technology (still in use as of 2019) for producing influenza vaccine was created in the 1950s.[191] In the US swine flu scare of 1976, President Gerald Ford was confronted with a potential swine flu pandemic. The vaccination program was rushed, yet plagued by delays and public relations problems. Meanwhile, maximum military containment efforts succeeded unexpectedly in confining the new strain to the single army base where it had originated. On that base, a number of soldiers fell severely ill, but only one died. The program was canceled after about 24% of the population had received vaccinations. An excess in deaths of twenty-five over normal annual levels as well as 400 excess hospitalizations, both from Guillain–Barré syndrome, were estimated to have occurred from the vaccination program itself, illustrating that the vaccine itself is not free of risks. The result has been cited to stoke lingering doubts about vaccination.[192] In the end, however, even the maligned 1976 vaccine may have saved lives. A 2010 study found a significantly enhanced immune response against the 2009 pandemic H1N1 in study participants who had received vaccination against the swine flu in 1976.[193]
Quadrivalent vaccines for seasonal flu
A quadrivalent flu vaccine administered by nasal mist was approved by the FDA in March 2012.[194][195] Fluarix Quadrivalent was approved by the FDA in December 2012.[196]
In 2014, the Canadian National Advisory Committee on Immunization (NACI) published a review of quadrivalent influenza vaccines.[197]
Starting with the 2018-2019 influenza season most of the regular-dose egg-based flu shots and all of the recombinant and cell-grown flu vaccines in the United States are quadrivalent.[198] In the 2019–2020 influenza season all regular-dose flu shots and all recombinant influenza vaccine in the United States are quadrivalent.[40]
In November 2019, the FDA approved Fluzone High-Dose Quadrivalent for use in the United States starting with the 2020-2021 influenza season.[199][200]
Cost-effectiveness
The cost-effectiveness of seasonal influenza vaccination has been widely evaluated for different groups and in different settings.[201] In the elderly (aged over 65 years) the majority of published studies have found that vaccination is cost saving, with the cost savings associated with influenza vaccination (e.g. prevented healthcare visits) outweighing the cost of vaccination.[202] In older adults (aged 50–64 years), several published studies have found that influenza vaccination is likely to be cost-effective, however the results of these studies were often found to be dependent on key assumptions used in the economic evaluations.[203] The uncertainty in influenza cost-effectiveness models can partially be explained by the complexities involved in estimating the disease burden,[204] as well as the seasonal variability in the circulating strains and the match of the vaccine.[205][206] In healthy working adults (aged 18–49 years), a 2012 review found that vaccination was generally not cost-saving, with the suitability for funding being dependent on the willingness to pay to obtain the associated health benefits.[207] In children, the majority of studies have found that influenza vaccination was cost-effective, however many of the studies included (indirect) productivity gains, which may not be given the same weight in all settings.[208] Several studies have attempted to predict the cost-effectiveness of interventions (including prepandemic vaccination) to help protect against a future pandemic, however estimating the cost-effectiveness has been complicated by uncertainty as to the severity of a potential future pandemic and the efficacy of measures against it.[209]
Research
Influenza research includes molecular virology, molecular evolution, pathogenesis, host immune responses, genomics, and epidemiology. These help in developing influenza countermeasures such as vaccines, therapies and diagnostic tools. Improved influenza countermeasures require basic research on how viruses enter cells, replicate, mutate, evolve into new strains and induce an immune response. The Influenza Genome Sequencing Project is creating a library of influenza sequences[210] that will help researchers' understanding of what makes one strain more lethal than another, what genetic determinants most affect immunogenicity, and how the virus evolves over time. Solutions to limitations in current vaccine methods are being researched.
A different approach uses Internet content to estimate the impact of an influenza vaccination campaign. More specifically, researchers have used data from Twitter and Microsoft's Bing search engine, and proposed a statistical framework which, after a series of operations, maps this information to estimates of the influenza-like illness reduction percentage in areas where vaccinations have been performed. The method has been used to quantify the impact of two flu vaccination programmes in England (2013/14 and 2014/15), where school-age children were administered a live attenuated influenza vaccine (LAIV). Notably, the impact estimates were in accordance with estimations from Public Health England based on traditional syndromic surveillance endpoints.[211][212]
Rapid response to pandemic flu
The rapid development, production, and distribution of pandemic influenza vaccines could potentially save millions of lives during an influenza pandemic. Due to the short time frame between identification of a pandemic strain and need for vaccination, researchers are looking at novel technologies for vaccine production that could provide better "real-time" access and be produced more affordably, thereby increasing access for people living in low- and moderate-income countries, where an influenza pandemic may likely originate, such as live attenuated (egg-based or cell-based) technology and recombinant technologies (proteins and virus-like particles).[213] As of July 2009, more than 70 known clinical trials have been completed or are ongoing for pandemic influenza vaccines.[214] In September 2009, the FDA approved four vaccines against the 2009 H1N1 influenza virus (the 2009 pandemic strain), and expected the initial vaccine lots to be available within the following month.[215]
In January 2020, the US Food and Drug Administration (FDA) approved Audenz as a vaccine for the H5N1 flu virus.[216] Audenz is a vaccine indicated for active immunization for the prevention of disease caused by the influenza A virus H5N1 subtype contained in the vaccine. Audenz is approved for use in persons six months of age and older at increased risk of exposure to the influenza A virus H5N1 subtype contained in the vaccine.[217]
Universal flu vaccines
A "universal vaccine" that would not have to be designed and made for each flu season in each hemisphere would be useful, in order to stabilize the supply and to ensure against error in the design or escape of the circulating strains by mutation.[140] Such a vaccine has been the subject of research for decades.[218]
One promising approach is using broadly neutralizing antibodies that unlike the annual seasonal vaccines used over the last several decades which provoke the body to generate an immune response, instead provide a component of the immune response itself. The first neutralizing antibodies were identified in 1993, via experimentation;[219] with time researchers understood that the flu neutralizing antibodies were binding to the stalk of the Hemagglutinin protein; later researchers identified antibodies that could bind to the head of those proteins. Later yet, researchers identified the highly conserved M2 proton channel as a potential target for broadly neutralizing antibodies.[218][220]
The challenges for researchers have been identifying single antibodies that could neutralize many subtypes of the virus, so that they could be useful in any season, and that target conserved domains that are resistant to antigenic drift.[218]
Another approach has been taking the conserved domains identified from these projects, and delivering groups of these antigens to provoke an immune response; various approaches with different antigens, presented different ways (as fusion proteins, mounted on virus-like particles, on non-pathogenic viruses, as DNA, and others), are under development.[220][221][222]
Efforts have also been undertaken to develop universal vaccines that specifically activate a T-cell response, based on clinical data showing that people with a strong, early T-cell response have better outcomes when infected with influenza and because T-cells respond to conserved epitopes. The challenge for developers is that these epitopes are on internal protein domains that are only mildly immunogenic.[220]
Along with the rest of the vaccine field, people working on universal vaccines have been experimenting with vaccine adjuvants to improve the ability of their vaccines to create a sufficiently powerful and enduring immune response.[220][223]
There have been some clinical trials of the M-001[224][225][140][226][227] and H1ssF_3928 universal influenza vaccine candidates. As of May 2020, six of seven M-001 trials are completed. One of the seven M-001 trials has results. None of the BiondVax-sponsored M-001 trials have results.[140][228][229]
Oral influenza vaccine
As of 2019, an oral flu vaccine was in clinical research.[230] The oral vaccine candidate is based on an adenovirus type 5 vector modified to remove genes needed for replication, with an added gene that expresses a small double-stranded RNA hairpin molecule as an adjuvant.[231] In 2020, a phase II human trial of the pill form of the vaccine showed that it was well tolerated and provided similar immunity to a licensed injectable vaccine.[232]
Veterinary use
Veterinary influenza vaccination aims to achieve the following four objectives:[233]
- Protection from clinical disease
- Protection from infection with virulent virus
- Protection from virus excretion
- Serological differentiation of infected from vaccinated animals (so-called DIVA principle).
Horses
Horses with horse flu can run a fever, have a dry hacking cough, have a runny nose, and become depressed and reluctant to eat or drink for several days but usually recover in two to three weeks. "Vaccination schedules generally require a primary course of two doses, 3–6 weeks apart, followed by boosters at 6–12 month intervals. It is generally recognized that in many cases such schedules may not maintain protective levels of antibody and more frequent administration is advised in high-risk situations."[234]
It is a common requirement at shows in the United Kingdom that horses be vaccinated against equine flu and a vaccination card must be produced; the International Federation for Equestrian Sports (FEI) requires vaccination every six months.[235][236]
Poultry
Poultry vaccines for bird flu are made inexpensively and are not filtered and purified like human vaccines to remove bits of bacteria or other viruses. They usually contain whole virus, not just hemagglutinin as in most human flu vaccines. Another difference between human and poultry vaccines is that poultry vaccines are adjuvated with mineral oil, which induces a strong immune reaction but can cause inflammation and abscesses. "Chicken vaccinators who have accidentally jabbed themselves have developed painful swollen fingers or even lost thumbs, doctors said. Effectiveness may also be limited. Chicken vaccines are often only vaguely similar to circulating flu strains – some contain an H5N2 strain isolated in Mexico years ago. 'With a chicken, if you use a vaccine that's only 85 percent related, you'll get protection,' Dr. Cardona said. 'In humans, you can get a single point mutation, and a vaccine that's 99.99 percent related won't protect you.' And they are weaker [than human vaccines]. 'Chickens are smaller and you only need to protect them for six weeks, because that's how long they live till you eat them,' said Dr. John J. Treanor, a vaccine expert at the University of Rochester. Human seasonal flu vaccines contain about 45 micrograms of antigen, while an experimental A(H5N1) vaccine contains 180. Chicken vaccines may contain less than one microgram. 'You have to be careful about extrapolating data from poultry to humans,' warned Dr. David E. Swayne, director of the agriculture department's Southeast Poultry Research Laboratory. 'Birds are more closely related to dinosaurs.'"[237]
Researchers, led by Nicholas Savill of the University of Edinburgh in Scotland, used mathematical models to simulate the spread of H5N1 and concluded that "at least 95 percent of birds need to be protected to prevent the virus spreading silently. In practice, it is difficult to protect more than 90 percent of a flock; protection levels achieved by a vaccine are usually much lower than this."[238] The Food and Agriculture Organization of the United Nations has issued recommendations on the prevention and control of avian influenza in poultry, including the use of vaccination.[239]
A filtered and purified Influenza A vaccine for humans is being developed and many countries have recommended it be stockpiled so if an Avian influenza pandemic starts jumping to humans, the vaccine can quickly be administered to avoid loss of life. Avian influenza is sometimes called avian flu, and commonly bird flu.[240]
Pigs
Swine influenza vaccines are extensively used in pig farming in Europe and North America. Most swine flu vaccines include an H1N1 and an H3N2 strain.
Swine influenza has been recognized as a major problem since the outbreak in 1976. Evolution of the virus has resulted in inconsistent responses to traditional vaccines. Standard commercial swine flu vaccines are effective in controlling the problem when the virus strains match enough to have significant cross-protection. Customised (autogenous) vaccines made from the specific viruses isolated, are made and used in the more difficult cases.[241] The vaccine manufacturer Novartis claims that the H3N2 strain (first identified in 1998) has brought major losses to pig farmers. Abortion storms are a common sign and sows stop eating for a few days and run a high fever. The mortality rate can be as high as 15 percent.[242]
Dogs
In 2004, influenza A virus subtype H3N8 was discovered to cause canine influenza. Because of the lack of previous exposure to this virus, dogs have no natural immunity to this virus. However, a vaccine was found in 2004.[243]
Notes
- (H1N1)pdm09 is newer nomenclature for the 2009 pandemic H1N1 virus, not a different strain.
References
- "Influenza virus vaccine, inactivated Use During Pregnancy". Drugs.com. July 10, 2019. Retrieved January 25, 2020.
- World Health Organization (November 2012). "Vaccines against influenza WHO position paper". Weekly Epidemiological Record. 87 (47): 461–76. hdl:10665/241993. PMID 23210147. Lay summary (PDF).
- Manzoli L, Ioannidis JP, Flacco ME, De Vito C, Villari P (July 2012). "Effectiveness and harms of seasonal and pandemic influenza vaccines in children, adults and elderly: a critical review and re-analysis of 15 meta-analyses". Human Vaccines & Immunotherapeutics. 8 (7): 851–62. doi:10.4161/hv.19917. PMC 3495721. PMID 22777099.
- Rolfes MA, Foppa IM, Garg S, Flannery B, Brammer L, Singleton JA, et al. (December 9, 2016). "2015–2016 Estimated Influenza Illnesses, Medical visits, and Hospitalizations Averted by Vaccination in the United States". U.S. Centers for Disease Control and Prevention (CDC). Archived from the original on December 2, 2019. Retrieved December 24, 2017.

- Centers for Disease Control and Prevention (January 16, 2020). "Benefits of Flu Vaccination During 2018-2019 Flu Season". Centers for Disease Control and Prevention (CDC). Retrieved April 10, 2020.
- Demicheli V, Jefferson T, Ferroni E, Rivetti A, Di Pietrantonj C (February 2018). "Vaccines for preventing influenza in healthy adults". Cochrane Database of Systematic Reviews. 2: CD001269. doi:10.1002/14651858.CD001269.pub6. PMC 6491184. PMID 29388196.
- Osterholm MT, Kelley NS, Sommer A, Belongia EA (January 2012). "Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis". The Lancet. Infectious Diseases. 12 (1): 36–44. doi:10.1016/S1473-3099(11)70295-X. PMID 22032844.
- Demicheli V, Jefferson T, Di Pietrantonj C, Ferroni E, Thorning S, Thomas RE, Rivetti A (February 2018). "Vaccines for preventing influenza in the elderly". Cochrane Database of Systematic Reviews. 2: CD004876. doi:10.1002/14651858.CD004876.pub4. PMC 6491101. PMID 29388197.
- "Who Should and Who Should NOT get a Flu Vaccine". U.S. Centers for Disease Control and Prevention (CDC). October 11, 2019. Archived from the original on December 2, 2019. Retrieved December 2, 2019.

- World Health Organization (October 2017). The immunological basis for immunization series: module 23: influenza vaccines. World Health Organization (WHO). hdl:10665/259211. ISBN 978-9241513050.
- Grohskopf LA, Alyanak E, Broder KR, Walter EB, Fry AM, Jernigan DB (August 2019). "Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices – United States, 2019–20 Influenza Season" (PDF). MMWR Recomm Rep. 68 (3): 1–21. doi:10.15585/mmwr.rr6803a1. PMC 6713402. PMID 31441906. Lay summary.

- "Implementation of the Council Recommendation on seasonal influenza vaccination (2009/1019/EU)" (PDF). European Centre for Disease Prevention and Control. January 2014. Retrieved April 10, 2020. Lay summary.
- "Flu Vaccine and People with Egg Allergies". U.S. Centers for Disease Control and Prevention (CDC). November 25, 2019. Archived from the original on December 2, 2019. Retrieved December 2, 2019.

- "Intradermal Influenza (Flu) Vaccination". U.S. Centers for Disease Control and Prevention (CDC). October 31, 2018. Archived from the original on October 14, 2019. Retrieved October 14, 2019.

- "Influenza vaccines – United States, 2019–20 influenza season". U.S. Centers for Disease Control and Prevention (CDC). August 22, 2019. Archived from the original on October 14, 2019. Retrieved October 14, 2019.

- "Influenza Virus Vaccine Inactivated". The American Society of Health-System Pharmacists. November 19, 2018. Archived from the original on October 14, 2019. Retrieved October 13, 2019.
- Compans RW (2009). Vaccines for pandemic influenza. Dordrecht: Springer. p. 49. ISBN 978-3540921653.
- Vaccine Analysis: Strategies, Principles, and Control. Springer. 2014. p. 61. ISBN 978-3662450246.
- World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- "Key Facts About Seasonal Flu Vaccine". U.S. Centers for Disease Control and Prevention (CDC). December 2, 2019. Archived from the original on December 2, 2019. Retrieved December 2, 2019.

- Osterholm MT, Kelley NS, Sommer A, Belongia EA (January 2012). "Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis". The Lancet. Infectious Diseases. 12 (1): 36–44. doi:10.1016/s1473-3099(11)70295-x. PMID 22032844.
- MacIntyre, CR; Mahimbo, A; Moa, AM; Barnes, M (December 15, 2016). "Influenza vaccine as a coronary intervention for prevention of myocardial infarction". Heart (British Cardiac Society). 102 (24): 1953–56. doi:10.1136/heartjnl-2016-309983. PMC 5256393. PMID 27686519.
- "Past Seasons Vaccine Effectiveness Estimates". U.S. Centers for Disease Control and Prevention (CDC). January 29, 2020. Archived from the original on February 12, 2020. Retrieved March 4, 2019.

- Doyle JD, Chung JR, Kim SS, Gaglani M, Raiyani C, Zimmerman RK, et al. (February 2019). "Interim Estimates of 2018-19 Seasonal Influenza Vaccine Effectiveness - United States, February 2019" (PDF). Morbidity and Mortality Weekly Report (MMWR). 68 (6): 135–39. doi:10.15585/mmwr.mm6806a2. PMC 6375657. PMID 30763298.

- Dawood FS, Chung JR, Kim SS, Zimmerman RK, Nowalk MP, Jackson ML, et al. (February 2020). "Interim Estimates of 2019-20 Seasonal Influenza Vaccine Effectiveness - United States, February 2020" (PDF). Morbidity and Mortality Weekly Report (MMWR). 69 (7): 177–82. doi:10.15585/mmwr.mm6907a1. PMC 7043386. PMID 32078591.

- Fedson DS (1998). "Measuring protection: efficacy versus effectiveness". Developments in Biological Standardization. 95: 195–201. PMID 9855432.
- Stephenson I, Zambon MC, Rudin A, Colegate A, Podda A, Bugarini R, et al. (May 2006). "Phase I evaluation of intranasal trivalent inactivated influenza vaccine with nontoxigenic Escherichia coli enterotoxin and novel biovector as mucosal adjuvants, using adult volunteers". Journal of Virology. 80 (10): 4962–70. doi:10.1128/JVI.80.10.4962-4970.2006. PMC 1472052. PMID 16641287.
- Jefferson T (October 2006). "Influenza vaccination: policy versus evidence". BMJ. 333 (7574): 912–15. doi:10.1136/bmj.38995.531701.80. PMC 1626345. PMID 17068038.
- "2007–2008 Influenza (Flu) Season". U.S. Centers for Disease Control and Prevention (CDC). June 26, 2008. Archived from the original on March 6, 2008.

- Carrat, F.; Flahault, A. (September 28, 2007). "Influenza vaccine: The challenge of antigenic drift". Vaccine. 25 (39): 6852–62. doi:10.1016/j.vaccine.2007.07.027. ISSN 0264-410X. PMID 17719149.
- "Vaccine Effectiveness: How Well Do the Flu Vaccines Work?". U.S. Centers for Disease Control and Prevention (CDC). October 12, 2018. Archived from the original on October 25, 2019. Retrieved October 24, 2019.

- Ramsay LC, Buchan SA, Stirling RG, Cowling BJ, Feng S, Kwong JC, et al. (January 2019). "The impact of repeated vaccination on influenza vaccine effectiveness: a systematic review and meta-analysis". BMC Med. 17 (1): 9. doi:10.1186/s12916-018-1239-8. PMC 6327561. PMID 30626399.
- Belongia EA, Skowronski DM, McLean HQ, Chambers C, Sundaram ME, De Serres G (July 2017). "Repeated annual influenza vaccination and vaccine effectiveness: review of evidence". Expert Rev Vaccines. 16 (7): 723–36. doi:10.1080/14760584.2017.1334554. PMID 28562111.
- Brownlee S (November 1, 2009). "Does the Vaccine Matter?". The Atlantic. Archived from the original on December 9, 2014. Retrieved December 8, 2014.
- Rabin RC (November 5, 2012). "Reassessing Flu Shots as the Season Draws Near". The New York Times. Archived from the original on November 10, 2016. Retrieved December 30, 2016.
'We have overpromoted and overhyped this vaccine,' said Michael T. Osterholm, director of the Center for Infectious Disease Research and Policy, as well as its Center of Excellence for Influenza Research and Surveillance. 'It does not protect as promoted. It's all a sales job: it's all public relations.'
- "Influenza (Seasonal)". World Health Organization (WHO). November 6, 2018. Archived from the original on October 25, 2019. Retrieved October 24, 2019.
- "Study of Flu-Related Deaths in Children Shows Healthy Children at Risk". U.S. Centers for Disease Control and Prevention (CDC). February 12, 2018. Archived from the original on December 2, 2019. Retrieved December 2, 2019.

- Jefferson T, Rivetti A, Di Pietrantonj C, Demicheli V (February 2018). "Vaccines for preventing influenza in healthy children". Cochrane Database of Systematic Reviews. 2: CD004879. doi:10.1002/14651858.CD004879.pub5. PMC 6491174. PMID 29388195.
- Steenhuysen J (January 22, 2018). "U.S. CDC director urges flu vaccinations as pediatric deaths mount". Reuters. Retrieved January 26, 2018.
- Centers for Disease Control and Prevention (CDC), National Center for Immunization and Respiratory Diseases (NCIRD) (November 5, 2019). "Frequently Asked Influenza (Flu) Questions: 2019–2020 Season". U.S. Centers for Disease Control and Prevention (CDC). Archived from the original on December 1, 2019. Retrieved November 30, 2019.

- "Fluzone High-Dose Quadrivalent". U.S. Food and Drug Administration (FDA). November 14, 2019. Archived from the original on December 1, 2019. Retrieved November 30, 2019.

- "Afluria Quadrivalent". U.S. Food and Drug Administration (FDA). November 8, 2019. Archived from the original on December 1, 2019. Retrieved November 30, 2019.

- National Advisory Committee on Immunization (NACI) (November 2018). "Supplemental Statement – Afluria Tetra – An Advisory Committee Statement (ACS)". Public Health Agency of Canada. Ottawa. Cat.: HP37-25E-PDF Pub.: 180566. Retrieved January 11, 2020.
- Literature review on influenza vaccination in healthy 5–18-year-olds (PDF) (Report). Ottawa: Public Health Agency of Canada. July 2014. ISBN 978-1-100-24681-9. Cat.: HP40-116/2014E-PDF Pub.: 140116.
- Literature Review on Pediatric Fluad Influenza Vaccine Use in Children 6–72 Months of Age (PDF) (Report). Ottawa: Public Health Agency of Canada. 2015. Retrieved January 11, 2020.
- Peleg, Noam; Zevit, Noam; Shamir, Raanan; Chodick, Gabriel; Levy, Itzhak (January 2015). "Seasonal influenza vaccination rates and reasons for non-vaccination in children with gastrointestinal disorders". Vaccine. 33 (1): 182–86. doi:10.1016/j.vaccine.2014.10.086.
- Burls A, Jordan R, Barton P, Olowokure B, Wake B, Albon E, Hawker J (May 2006). "Vaccinating healthcare workers against influenza to protect the vulnerable – is it a good use of healthcare resources? A systematic review of the evidence and an economic evaluation". Vaccine. 24 (19): 4212–21. doi:10.1016/j.vaccine.2005.12.043. PMID 16546308.
- Ahmed F, Lindley MC, Allred N, Weinbaum CM, Grohskopf L (January 2014). "Effect of influenza vaccination of healthcare personnel on morbidity and mortality among patients: systematic review and grading of evidence". Clinical Infectious Diseases. 58 (1): 50–57. doi:10.1093/cid/cit580. PMID 24046301.
- Griffin MR (January 2014). "Influenza vaccination of healthcare workers: making the grade for action". Clinical Infectious Diseases. 58 (1): 58–60. doi:10.1093/cid/cit590. PMID 24046312.
- Simonsen L, Viboud C, Taylor RJ, Miller MA, Jackson L (October 2009). "Influenza vaccination and mortality benefits: new insights, new opportunities". Vaccine. 27 (45): 6300–04. doi:10.1016/j.vaccine.2009.07.008. PMID 19840664.
- Darvishian M, Bijlsma MJ, Hak E, van den Heuvel ER (December 2014). "Effectiveness of seasonal influenza vaccine in community-dwelling elderly people: a meta-analysis of test-negative design case-control studies". The Lancet. Infectious Diseases. 14 (12): 1228–39. doi:10.1016/S1473-3099(14)70960-0. PMID 25455990.
- Nichol KL, Nordin JD, Nelson DB, Mullooly JP, Hak E (October 2007). "Effectiveness of influenza vaccine in the community-dwelling elderly". The New England Journal of Medicine. 357 (14): 1373–81. doi:10.1056/NEJMoa070844. PMID 17914038.
- Simonsen L, Taylor RJ, Viboud C, Miller MA, Jackson LA (October 2007). "Mortality benefits of influenza vaccination in elderly people: an ongoing controversy". The Lancet. Infectious Diseases. 7 (10): 658–66. doi:10.1016/S1473-3099(07)70236-0. PMID 17897608.
- Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K (January 2003). "Mortality associated with influenza and respiratory syncytial virus in the United States". JAMA. 289 (2): 179–86. doi:10.1001/jama.289.2.179. PMID 12517228.
- "High Dose Flu Vaccine for the Elderly « Science-Based Medicine". Sciencebasedmedicine.org. Archived from the original on May 8, 2013. Retrieved October 17, 2013.
- "Fluzone High-Dose Seasonal Influenza Vaccine". U.S. Centers for Disease Control and Prevention (CDC). September 6, 2019. Archived from the original on December 2, 2019. Retrieved December 2, 2019.
- DiazGranados CA, Dunning AJ, Kimmel M, Kirby D, Treanor J, Collins A, et al. (August 2014). "Efficacy of high-dose versus standard-dose influenza vaccine in older adults". The New England Journal of Medicine. 371 (7): 635–45. doi:10.1056/NEJMoa1315727. PMID 25119609.
- Wells C, Grobelna A (January 8, 2019). "High Dose Influenza Vaccine for Adults: A Review of Clinical Effectiveness, Cost-Effectiveness, and Guidelines" (PDF). Rapid Response Report. Ottawa: Canadian Agency for Drugs and Technologies in Health (CADTH). ISSN 1922-8147. PMID 31141324.
- Mascagni P, Vicenzi E, Kajaste-Rudnitski A, Pellicciotta G, Monti A, Cervi C, et al. (2012). "Assessment of efficacy and safety of pandemic A/H1N1/2009 influenza vaccine in a group of health care workers". La Medicina del Lavoro. 103 (3): 220–29. PMID 22838300.
- "FDA approves first seasonal influenza vaccine containing an adjuvant" (Press release). U.S. Food and Drug Administration. November 24, 2015. Archived from the original on July 22, 2017. Retrieved August 20, 2017.

- "Flu Vaccine With Adjuvant". U.S. Centers for Disease Control and Prevention (CDC). September 4, 2019. Archived from the original on December 2, 2019. Retrieved December 2, 2019.

- "Fluad". U.S. Food and Drug Administration (FDA). November 8, 2019. STN 125510. Archived from the original on December 2, 2019. Retrieved December 2, 2019.

- "Influenza vaccine with squalene adjuvant: new preparation. No better than available products". Prescrire International. 13 (74): 206–08. December 2004. PMID 15599987.
- Camilloni B, Basileo M, Valente S, Nunzi E, Iorio AM (2015). "Immunogenicity of intramuscular MF59-adjuvanted and intradermal administered influenza enhanced vaccines in subjects aged over 60: A literature review". Human Vaccines & Immunotherapeutics. 11 (3): 553–63. doi:10.1080/21645515.2015.1011562. PMC 4514405. PMID 25714138. Lay summary.
- Van Damme P, Arnou R, Kafeja F, Fiquet A, Richard P, Thomas S, et al. (May 2010). "Evaluation of non-inferiority of intradermal versus adjuvanted seasonal influenza vaccine using two serological techniques: a randomised comparative study". BMC Infectious Diseases. 10: 134. doi:10.1186/1471-2334-10-134. PMC 2895601. PMID 20504306.
- Haverkate M, D'Ancona F, Giambi C, Johansen K, Lopalco PL, Cozza V, Appelgren E, VENICE project gatekeepers contact points (May 2012). "Mandatory and recommended vaccination in the EU, Iceland and Norway: results of the VENICE 2010 survey on the ways of implementing national vaccination programmes". Euro Surveillance. 17 (22). doi:10.2807/ese.17.22.20183-en. PMID 22687916.
- Field RI (November 2009). "Mandatory vaccination of health care workers: whose rights should come first?". P & T. 34 (11): 615–18. PMC 2810172. PMID 20140133.
- Kassianos G (2015). "Willingness of European healthcare workers to undergo vaccination against seasonal influenza: current situation and suggestions for improvement". Drugs in Context. 4: 212268. doi:10.7573/dic.212268. PMC 4316812. PMID 25657810.
- Thomas RE, Jefferson T, Lasserson TJ (June 2016). "Influenza vaccination for healthcare workers who care for people aged 60 or older living in long-term care institutions". The Cochrane Database of Systematic Reviews (6): CD005187. doi:10.1002/14651858.CD005187.pub5. PMID 27251461.
- "Fluad Quadrivalent". U.S. Food and Drug Administration (FDA). February 21, 2020. Retrieved May 29, 2020.
- "Fluad Tetra EPAR". European Medicines Agency (EMA). March 24, 2020. Retrieved May 29, 2020.
- Fell DB, Sprague AE, Liu N, Yasseen AS, Wen SW, Smith G, Walker MC (June 2012). "H1N1 influenza vaccination during pregnancy and fetal and neonatal outcomes". American Journal of Public Health (Submitted manuscript). 102 (6): e33–40. doi:10.2105/AJPH.2011.300606. PMC 3483960. PMID 22515877.
- Madhi SA, Cutland CL, Kuwanda L, Weinberg A, Hugo A, Jones S, et al. (September 2014). "Influenza vaccination of pregnant women and protection of their infants". The New England Journal of Medicine. 371 (10): 918–31. doi:10.1056/NEJMoa1401480. hdl:2263/42412. PMID 25184864.
- Haber P, Sejvar J, Mikaeloff Y, DeStefano F (2009). "Vaccines and Guillain-Barré syndrome". Drug Safety. 32 (4): 309–23. doi:10.2165/00002018-200932040-00005. PMID 19388722.
- "Reorganized text". JAMA Otolaryngology–Head & Neck Surgery. 141 (5): 428. May 2015. doi:10.1001/jama.248.6.698. PMID 25996397.
- Stowe J, Andrews N, Wise L, Miller E (February 2009). "Investigation of the temporal association of Guillain-Barre syndrome with influenza vaccine and influenzalike illness using the United Kingdom General Practice Research Database". American Journal of Epidemiology. 169 (3): 382–88. doi:10.1093/aje/kwn310. PMID 19033158.
- Sivadon-Tardy V, Orlikowski D, Porcher R, Sharshar T, Durand MC, Enouf V, et al. (January 2009). "Guillain-Barré syndrome and influenza virus infection". Clinical Infectious Diseases. 48 (1): 48–56. doi:10.1086/594124. PMID 19025491.
- Vellozzi C, Burwen DR, Dobardzic A, Ball R, Walton K, Haber P (March 2009). "Safety of trivalent inactivated influenza vaccines in adults: background for pandemic influenza vaccine safety monitoring". Vaccine. 27 (15): 2114–20. doi:10.1016/j.vaccine.2009.01.125. PMID 19356614.
- Reinberg S (February 2, 2011). "Last Year's H1N1 Flu Vaccine Was Safe, Study Finds". U.S. News & World Report. Archived from the original on April 25, 2013.
- National Advisory Committee on Immunization (NACI) (August 2012). "Statement on Seasonal Influenza Vaccine for 2012–2013" (PDF). Canadian Communicable Disease Report. Ottawa. 38. Archived from the original (PDF) on January 17, 2013. Retrieved July 18, 2013.
- Turner PJ, Southern J, Andrews NJ, Miller E, Erlewyn-Lajeunesse M (December 2015). "Safety of live attenuated influenza vaccine in young people with egg allergy: multicentre prospective cohort study". BMJ. 351: h6291. doi:10.1136/bmj.h6291. PMC 4673102. PMID 26645895.
- Greenhawt M (December 2015). "Live attenuated influenza vaccine for children with egg allergy". BMJ. 351: h6656. doi:10.1136/bmj.h6656. PMID 26657778.
- Technical Report: Narcolepsy in association with pandemic influenza vaccination (PDF). Stockholm, Sweden: European Centre for Disease Prevention and Control (ECDC). 2012. ISBN 978-92-9193-388-4. Archived from the original (PDF) on December 31, 2013. Retrieved December 30, 2013.
- Yong E (2013). "Narcolepsy confirmed as autoimmune disease". Nature. doi:10.1038/nature.2013.14413.
- "Thimerosal in Flu Vaccine". U.S. Centers for Disease Control and Prevention. October 16, 2015. Archived from the original on December 2, 2019. Retrieved December 2, 2019.

- "Thimerosal in Vaccines Thimerosal – Concerns – Vaccine Safety". U.S. Centers for Disease Control and Prevention. October 27, 2015. Archived from the original on November 2, 2019. Retrieved December 2, 2019.

- Offit PA (September 2007). "Thimerosal and vaccines – a cautionary tale". The New England Journal of Medicine. 357 (13): 1278–79. doi:10.1056/NEJMp078187. PMID 17898096.
- Global Advisory Committee on Vaccine Safety (July 14, 2006). "Thiomersal and vaccines". World Health Organization (WHO). Archived from the original on November 6, 2009. Retrieved November 20, 2007.
- Plotkin & Mortimer (1988). Vaccines. Philadelphia: W.B. Saunders Company. ISBN 978-0-7216-1946-0.
- Product Monograph: Flumist, Astrazeneca Canada Inc., 2011
- Fiore AE, Shay DK, Broder K, Iskander JK, Uyeki TM, Mootrey G, et al. (July 2009). "Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009" (PDF). Recommendations and Reports (MMWR). 58 (RR-8): 1–52. PMID 19644442. Archived (PDF) from the original on June 20, 2017.

- To KW, Lai A, Lee KC, Koh D, Lee SS (October 2016). "Increasing the coverage of influenza vaccination in healthcare workers: review of challenges and solutions". The Journal of Hospital Infection. 94 (2): 133–42. doi:10.1016/j.jhin.2016.07.003. PMID 27546456.
- Rubin GJ, Potts HW, Michie S (March 2011). "Likely uptake of swine and seasonal flu vaccines among healthcare workers. A cross-sectional analysis of UK telephone survey data". Vaccine. 29 (13): 2421–28. doi:10.1016/j.vaccine.2011.01.035. PMID 21277402.
- Skowronski DM, De Serres G (July 2009). "Is routine influenza immunization warranted in early pregnancy?". Vaccine. 27 (35): 4754–70. doi:10.1016/j.vaccine.2009.03.079. PMID 19515466.
- Institute for Clinical Systems Improvement (July 2010). "Health Care Guideline: Routine Prenatal Care" (Fourteenth ed.). Archived from the original on July 5, 2008.
- "Seasonal Flu Shot". U.S. Centers for Disease Control and Prevention (CDC). December 9, 2019. Retrieved January 12, 2020.

- "Vaccine use". World Health Organization (WHO). Archived from the original on December 15, 2012. Retrieved January 15, 2017.
- "Influenza (Seasonal) Fact sheet". World Health Organization (WHO). November 2016. Archived from the original on November 30, 2014. Retrieved January 15, 2017.
- "Influenza (Seasonal)". WHO. November 6, 2018. Archived from the original on October 14, 2019. Retrieved October 14, 2019.
- "Methods for assessing influenza vaccination coverage in target groups (2016)". WHO/Europe. March 19, 2018. Retrieved October 14, 2019.
- "Recommendations on influenza vaccination during the 2019–2020 winter season". WHO/Europe. September 24, 2019. Retrieved October 14, 2019.
- Canadian Immunization Guide Chapter on Influenza and Statement on Seasonal Influenza Vaccine for 2019–2020 (PDF) (Report). Public Health Agency of Canada. May 2019. Cat.: HP37-25E-PDF; Pub.: 180883. Lay summary.
- "Risk groups for severe influenza". European Centre for Disease Prevention and Control (ECDC). Archived from the original on October 22, 2019. Retrieved October 22, 2019.
- "ECDC Reviews – New WHO recommendations on seasonal influenza..." European Centre for Disease Prevention and Control (ECDC). Archived from the original on May 10, 2017. Retrieved December 25, 2016.
- "ECDC Guidance: Priority risk groups for influenza vaccination" (PDF). European Centre for Disease Prevention and Control (ECDC). pp. 7–8. Archived from the original (PDF) on December 25, 2016. Retrieved December 25, 2016.
- Fiore AE, Uyeki TM, Broder K, Finelli L, Euler GL, Singleton JA, et al. (August 2010). "Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010" (PDF). Recommendations and Reports (MMWR). 59 (RR-8): 1–62. PMID 20689501. Archived (PDF) from the original on September 21, 2012.

- Centers for Disease Control Prevention (CDC) (August 2012). "Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP) – United States, 2012–13 influenza season" (PDF). Morbidity and Mortality Weekly Report (MMWR). 61 (32): 613–18. PMID 22895385. Archived (PDF) from the original on January 23, 2013.

- "Children & Influenza (Flu)". U.S. Centers for Disease Control and Prevention (CDC). October 23, 2019. Archived from the original on November 11, 2019.

- "Live Attenuated Influenza Vaccine [LAIV] (The Nasal Spray Flu Vaccine)". U.S. Centers for Disease Control and Prevention (CDC). September 16, 2019. Archived from the original on October 14, 2019. Retrieved October 14, 2019.

- "Influenza Vaccination: A Summary for Clinicians – Health Professionals – Seasonal Influenza (Flu)". U.S. Centers for Disease Control and Prevention (CDC). September 6, 2018. Archived from the original on February 24, 2008.

- "Fluzone, Fluzone High-Dose and Fluzone Intradermal". U.S. Food and Drug Administration (FDA). July 11, 2017. Archived from the original on July 22, 2017. Retrieved June 1, 2020.CS1 maint: unfit url (link)
- Couch RB, Winokur P, Brady R, Belshe R, Chen WH, Cate TR, et al. (November 2007). "Safety and immunogenicity of a high dosage trivalent influenza vaccine among elderly subjects". Vaccine. 25 (44): 7656–63. doi:10.1016/j.vaccine.2007.08.042. PMC 2243220. PMID 17913310.
- Lee JK, Lam GK, Shin T, Kim J, Krishnan A, Greenberg DP, et al. (May 2018). "Efficacy and effectiveness of high-dose versus standard-dose influenza vaccination for older adults: a systematic review and meta-analysis". Expert Rev Vaccines. 17 (5): 435–443. doi:10.1080/14760584.2018.1471989. PMID 29715054.
- Robertson CA, DiazGranados CA, Decker MD, Chit A, Mercer M, Greenberg DP (December 2016). "Fluzone High-Dose Influenza Vaccine". Expert Rev Vaccines. 15 (12): 1495–1505. doi:10.1080/14760584.2016.1254044. PMID 27813430.
- Literature review update on the efficacy and effectiveness of high-dose (Fluzone High-Dose) and MF59-adjuvanted (Fluad) trivalent inactivated influenza vaccines in adults 65 years of age and older (PDF) (Report). Ottawa: Public Health Agency of Canada. May 2018. HP40-210/2018E-PDF. Retrieved June 1, 2020.
- Tanner L (January 13, 2013). "Hospitals crack down on workers who refuse flu shots". NBC News. Archived from the original on December 3, 2013. Retrieved July 24, 2014.
- Centers for Disease Control Prevention (CDC) (December 2010). "Seasonal influenza and 2009 H1N1 influenza vaccination coverage among pregnant women – 10 states, 2009–10 influenza season" (PDF). Morbidity and Mortality Weekly Report (MMWR). 59 (47): 1541–45. PMID 21124293. Archived (PDF) from the original on June 25, 2017.

- Altman LK (February 28, 2008). "Panel Advises Flu Shots for Children Up to Age 18". The New York Times. Archived from the original on January 22, 2015.
- "ACIP votes down use of LAIV for 2016–2017 flu season" (Press release). U.S. Centers for Disease Control and Prevention (CDC). June 22, 2016. Archived from the original on November 25, 2016. Retrieved November 26, 2016.

- "Immunization Schedules". U.S. Centers for Disease Control and Prevention (CDC). Archived from the original on November 5, 2014. Retrieved November 4, 2014.

- "Flu vaccine FAQs". Healthdirect, Department of Health, Government of Australia. April 1, 2019. Retrieved May 29, 2019.
- "Influenza fact sheet". Healthdirect, Department of Health, Government of Australia. April 1, 2019. Retrieved May 29, 2019.
- "Health care use - Influenza vaccination rates - OECD Data". theOECD. Retrieved April 24, 2020.
- Han YK, Michie S, Potts HW, Rubin GJ (March 2016). "Predictors of influenza vaccine uptake during the 2009/10 influenza A H1N1v ('swine flu') pandemic: Results from five national surveys in the United Kingdom". Preventive Medicine. 84: 57–61. doi:10.1016/j.ypmed.2015.12.018. PMC 4766366. PMID 26757401.
- Bish A, Yardley L, Nicoll A, Michie S (September 2011). "Factors associated with uptake of vaccination against pandemic influenza: a systematic review". Vaccine. 29 (38): 6472–84. doi:10.1016/j.vaccine.2011.06.107. PMID 21756960.
- Brien S, Kwong JC, Buckeridge DL (February 2012). "The determinants of 2009 pandemic A/H1N1 influenza vaccination: a systematic review". Vaccine. 30 (7): 1255–64. doi:10.1016/j.vaccine.2011.12.089. PMID 22214889.
- Thomas RE, Lorenzetti DL (May 2018). "Interventions to increase influenza vaccination rates of those 60 years and older in the community". The Cochrane Database of Systematic Reviews. 5: CD005188. doi:10.1002/14651858.CD005188.pub4. PMC 6494593. PMID 29845606.
- Centers for Disease Control Prevention (CDC) (October 2009). "Update on influenza A (H1N1) 2009 monovalent vaccines" (PDF). Morbidity and Mortality Weekly Report (MMWR). 58 (39): 1100–01. PMID 19816398. Archived (PDF) from the original on June 29, 2011.

- La Torre G, Di Thiene D, Cadeddu C, Ricciardi W, Boccia A (December 2009). "Behaviours regarding preventive measures against pandemic H1N1 influenza among Italian healthcare workers, October 2009". Euro Surveillance. 14 (49). PMID 20003908.
- Amodio E, Anastasi G, Marsala MG, Torregrossa MV, Romano N, Firenze A (February 2011). "Vaccination against the 2009 pandemic influenza A (H1N1) among healthcare workers in the major teaching hospital of Sicily (Italy)". Vaccine. 29 (7): 1408–12. doi:10.1016/j.vaccine.2010.12.041. PMID 21199700.
- Chor JS, Ngai KL, Goggins WB, Wong MC, Wong SY, Lee N, et al. (August 2009). "Willingness of Hong Kong healthcare workers to accept pre-pandemic influenza vaccination at different WHO alert levels: two questionnaire surveys". BMJ. 339: b3391. doi:10.1136/bmj.b3391. PMC 2731837. PMID 19706937.
- Schnirring L (August 18, 2011). "CDC updates flu vaccination recommendations". Center for Infectious Disease Research and Policy (CIDRAP). Archived from the original on October 25, 2019. Retrieved October 24, 2019.
- Centers for Disease Control Prevention (CDC) (August 2011). "Influenza vaccination coverage among health-care personnel – United States, 2010–11 influenza season" (PDF). Morbidity and Mortality Weekly Report (MMWR). 60 (32): 1073–77. PMID 21849963. Archived (PDF) from the original on May 25, 2017.

- Centers for Disease Control Prevention (CDC) (September 2012). "Influenza vaccination coverage among health-care personnel: 2011–12 influenza season, United States" (PDF). Morbidity and Mortality Weekly Report (MMWR). 61: 753–57. PMID 23013720. Archived (PDF) from the original on June 24, 2017.

- Bull AL, Bennett N, Pitcher HC, Russo PL, Richards MJ (February 2007). "Influenza vaccine coverage among health care workers in Victorian public hospitals". The Medical Journal of Australia. 186 (4): 185–86. doi:10.5694/j.1326-5377.2007.tb00858.x. PMID 17309419.
- "how it's made" (PDF). Archived from the original (PDF) on July 5, 2010.
- Greenfieldboyce N (November 8, 2007). New and Old Ways to Make Flu Vaccines (Radio broadcast). NPR. Archived from the original on October 24, 2019. Retrieved October 23, 2019.
- "Influenza vaccine viruses and reagents". World Health Organization (WHO). Archived from the original on May 27, 2013.
- "Recommendations for the production and control of influenza vaccine (inactivated)" (PDF). World Health Organization (WHO). Archived (PDF) from the original on October 28, 2013. Retrieved May 27, 2013.
- Abbasi J (November 2019). "The Search for a Universal Flu Vaccine Heats Up". JAMA. 322 (20): 1942. doi:10.1001/jama.2019.16816. PMID 31693060.
- "Priming with DNA vaccine makes avian flu vaccine work better (NIH News)". October 3, 2011. Archived from the original on September 27, 2012.
- Clinical trial number NCT00776711 for "Vaccine for Prevention of Bird Flu" at ClinicalTrials.gov
- Clinical trial number NCT01086657 for "An Open-Label, Randomized Phase I Study in Healthy Adults of the Safety and Immunogenicity of Prime-Boost Intervals with Monovalent Influenza Subunit Virion (H5N1) Vaccine, A/Indonesia/05/2005 (Sanofi Pasteur, Inc), Administered Alone or Following Recombinant DNA Plasmid (H5) Vaccine, VRC-AVIDNA036-00-VP (VRC, NIAID)" at ClinicalTrials.gov
- "Novartis receives FDA approval for Flucelvax, the first cell-culture vaccine in US to help protect against seasonal influenza" (Press release). Novartis. November 20, 2012. Archived from the original on November 28, 2012.
- "FDA approves first seasonal influenza vaccine manufactured using cell culture technology" (Press release). U.S. Food and Drug Administration (FDA). Archived from the original on January 2, 2013.

- Research, Center for Biologics Evaluation and. "Approved Products – November 20, 2012 Approval Letter- Flucelvax". U.S. Food and Drug Administration (FDA). Archived from the original on December 3, 2012.

- "Seqirus receives FDA approval for Flucelvax Quadrivalent (Influenza Vaccine) for people four years of age and older" (Press release). Seqirus. May 23, 2016. Archived from the original on January 16, 2017. Retrieved January 15, 2017.
- "Influenza Vaccine Strategies for Broad Global Access". Path. October 2007. Archived from the original on October 14, 2019. Retrieved September 16, 2009.
- Bright RA, Carter DM, Daniluk S, Toapanta FR, Ahmad A, Gavrilov V, et al. (May 2007). "Influenza virus-like particles elicit broader immune responses than whole virion inactivated influenza virus or recombinant hemagglutinin". Vaccine. 25 (19): 3871–78. doi:10.1016/j.vaccine.2007.01.106. PMID 17337102.
- Racaniello V (December 2009). "Influenza virus growth in eggs". Virology Blog. Archived from the original on December 25, 2014.
- Izzat F (April 2012). "Viral Cultivation in Chicken Embryo". Youtube. Archived from the original on May 26, 2015.
- "How Influenza (Flu) Vaccines Are Made". U.S. Centers for Disease Control and Prevention (CDC). November 26, 2019. Archived from the original on December 2, 2019. Retrieved December 2, 2019.

- "FDA approves new seasonal influenza vaccine made using novel technology" (Press release). U.S. Food and Drug Administration (FDA). January 16, 2013. Archived from the original on May 18, 2013.

- Roos R (October 14, 2019). "FDA approves first flu vaccine grown in insect cells". Center for Infectious Disease Research and Policy (CIDRAP). Archived from the original on October 14, 2019. Retrieved October 14, 2019.
- "Flublok". U.S. Food and Drug Administration (FDA). February 26, 2018. STN 125285. Archived from the original on October 14, 2019. Retrieved October 14, 2019.

- "Flublok Quadrivalent". U.S. Food and Drug Administration (FDA). August 2, 2019. STN 125285. Archived from the original on October 14, 2019. Retrieved October 14, 2019.

- "Global Influenza Surveillance and Response System (GISRS)". World Health Organization. Retrieved October 22, 2019.
- "Spotlight: Influenza". World Health Organization. Retrieved October 22, 2019.
- "Global influenza surveillance". WHO. Archived from the original on April 30, 2003.
- Brown D (February 18, 2008). "Keeping ahead of flu comes down to guessing game". Knoxville News Sentinel. The Washington Post. Archived from the original on January 11, 2009. Retrieved December 22, 2015.
- World Health Organization (2000). WHO Report on Global Surveillance of Epidemic-prone Infectious Diseases – Influenza. World Health Organization (WHO). hdl:10665/66485. WHO/CDS/CSR/ISR/2000/1.
- Grohskopf LA, Sokolow LZ, Broder KR, Walter EB, Bresee JS, Fry AM, et al. (August 2017). "Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices – United States, 2017–18 Influenza Season" (PDF). Recommendations and Reports (MMWR). 66 (2): 1–20. doi:10.15585/mmwr.rr6602a1. PMC 5837399. PMID 28841201.

- "WHO switches H1N1 in Southern Hemisphere flu vaccine". Center for Infectious Disease Research and Policy (CIDRAP). September 29, 2016. Retrieved September 12, 2017.
A/Michigan/45/2015 replaces the longstanding A/California/7/2009 and is recommended to improve protection against two subclades that have emerged over the past season.
- Karlamangla S (January 6, 2018). "Severe flu brings medicine shortages, packed ERs and a rising death toll in California". Los Angeles Times. Retrieved January 7, 2018.
- Sun LH (February 15, 2018). "This season's flu vaccine is only 36 percent effective, but experts say you should still get it". The Washington Post. Retrieved February 17, 2018.
- Flannery B, Chung JR, Belongia EA, McLean HQ, Gaglani M, Murthy K, et al. (February 2018). "Interim Estimates of 2017-18 Seasonal Influenza Vaccine Effectiveness - United States, February 2018" (PDF). Morbidity and Mortality Weekly Report (MMWR). 67 (6): 180–85. doi:10.15585/mmwr.mm6706a2. PMC 5815489. PMID 29447141.

- World Health Organization (October 2017). "Recommended composition of influenza virus vaccines for use in the 2018 southern hemisphere influenza season". Wkly. Epidemiol. Rec. 92 (42): 625–33. hdl:10665/259276. PMID 29052409.
- "Recommended composition of influenza virus vaccines for use in the 2018 southern hemisphere influenza season". World Health Organization (WHO). September 28, 2017. Retrieved September 28, 2017.
- World Health Organization (March 2018). "Recommended composition of influenza virus vaccines for use in the 2018–2019 northern hemisphere influenza season". Wkly. Epidemiol. Rec. 93 (12): 133–41. hdl:10665/272270. PMID 29569429.
- World Health Organization (2019). "Review of the 2018–2019 influenza season in the northern hemisphere". Wkly. Epidemiol. Rec. 94 (32): 345–63. hdl:10665/326244.
- "Recommended composition of influenza virus vaccines for use in the 2018–2019 northern hemisphere influenza season". World Health Organization (WHO). February 22, 2018. Retrieved September 12, 2018.
- "Influenza Virus Vaccine for the 2018–2019 Season". U.S. Food and Drug Administration. November 8, 2018. Archived from the original on December 10, 2019. Retrieved December 9, 2019.

- World Health Organization (2018). "Recommended composition of influenza virus vaccines for use in the 2019 southern hemisphere influenza season". Wkly. Epidemiol. Rec. 93 (42): 553–62. hdl:10665/275476.
- "Recommended composition of influenza virus vaccines for use in the 2019 southern hemisphere influenza season". World Health Organization (WHO). September 27, 2018. Retrieved September 27, 2018.
- "Standardization of terminology of the pandemic A(H1N1) 2009 virus" (PDF). World Health Organization (WHO). Retrieved June 14, 2012.
- "Recommended composition of influenza virus vaccines for use in the 2019–2020 northern hemisphere influenza season". World Health Organization (WHO). Retrieved March 22, 2019.
- "Influenza Vaccine for the 2019-2020 Season". U.S. Food and Drug Administration (FDA). December 5, 2019. Archived from the original on December 10, 2019. Retrieved December 9, 2019.

- "Vaccines and Related Biological Products Advisory Committee March 6–7, 2019 Meeting Announcement". U.S. Food and Drug Administration (FDA). March 6–7, 2019. Archived from the original on December 10, 2019. Retrieved December 9, 2019.

- "Vaccines and Related Biological Products Advisory Committee March 22, 2019 Meeting Announcement". U.S. Food and Drug Administration (FDA). March 22, 2019. Archived from the original on December 10, 2019. Retrieved December 9, 2019.

- "Influenza Vaccine for the 2020-2021 Season". U.S. Food and Drug Administration (FDA). July 10, 2020. Retrieved July 12, 2020.

- "EU recommendations for 2019/2020 seasonal flu vaccine composition". European Medicines Agency (EMA). May 15, 2019. Archived from the original on December 10, 2019. Retrieved December 9, 2019.
- World Health Organization (October 18, 2019). "Recommended composition of influenza virus vaccines for use in the 2020 southern hemisphere influenza season". Wkly. Epidemiol. Rec. 94 (42): 473–84. hdl:10665/329391.
- Institute of Medicine (2005). Knobler SL, Mack A, Mahmoud A, Lemon SM (eds.). The Threat of Pandemic Influenza: Are We Ready? Workshop Summary. The National Academies Press. p. 62. doi:10.17226/11150. ISBN 978-0-309-09504-4. PMID 20669448.
- Plotkin, S.L. and Plotkin, S.A. "A short history of vaccination." In: Vaccines, Stanley A. Plotkin, Walter A. Orenstein, Paul A. Offit, eds. Elsevier Health Sciences, 2008, pp. 6–7.
- Artenstein, A.W. "Influenza" In: Vaccines: A Biography Andrew W. Artenstein, ed. pp. 191–205.
- Hampson AW (June 2008). "Vaccines for pandemic influenza. The history of our current vaccines, their limitations and the requirements to deal with a pandemic threat". Annals of the Academy of Medicine, Singapore. 37 (6): 510–17. PMID 18618064.
- Milián E, Kamen AA (2015). "Current and emerging cell culture manufacturing technologies for influenza vaccines". Biomed Res Int. 2015: 504831. doi:10.1155/2015/504831. PMC 4359798. PMID 25815321.
- "Cell-Based Flu Vaccines". U.S. Centers for Disease Control and Prevention (CDC). October 11, 2019. Archived from the original on December 2, 2019. Retrieved December 2, 2019.

- Landry N, Ward BJ, Trépanier S, Montomoli E, Dargis M, Lapini G, Vézina LP (December 2010). "Preclinical and clinical development of plant-made virus-like particle vaccine against avian H5N1 influenza". PLOS ONE. 5 (12): e15559. Bibcode:2010PLoSO...515559L. doi:10.1371/journal.pone.0015559. PMC 3008737. PMID 21203523.
- Bridges CB, Fukuda K, Uyeki TM, Cox NJ, Singleton JA (April 2002). "Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practices (ACIP)" (PDF). Recommendations and Reports (MMWR). 51 (RR-3): 1–31. PMID 12002171. Archived (PDF) from the original on August 21, 2017.

- Osterholm MT (May 2005). "Preparing for the next pandemic". The New England Journal of Medicine. 352 (18): 1839–42. CiteSeerX 10.1.1.608.6200. doi:10.1056/NEJMp058068. PMID 15872196.
- "Swine Flu Epidemics". October 9, 1999. Archived from the original on October 9, 1999.
- McCullers JA, Van De Velde LA, Allison KJ, Branum KC, Webby RJ, Flynn PM (June 2010). "Recipients of vaccine against the 1976 "swine flu" have enhanced neutralization responses to the 2009 novel H1N1 influenza virus". Clinical Infectious Diseases. 50 (11): 1487–92. doi:10.1086/652441. PMC 2946351. PMID 20415539.
- "First Quadrivalent Vaccine Against Seasonal Flu Wins FDA Approval". Archived from the original on March 4, 2012.
- "FDA approves first quadrivalent vaccine to prevent seasonal influenza" (Press release). U.S. Food and Drug Administration (FDA). Archived from the original on December 21, 2012.

- "December 14, 2012 Approval Letter- Fluarix Quadrivalent". U.S. Food and Drug Administration (FDA). Archived from the original on January 2, 2013.

- National Advisory Committee on Immunization (NACI) (July 2014). "Literature review on quadrivalent influenza vaccines" (PDF). Public Health Agency of Canada. Ottawa. ISBN 978-1-100-24682-6. Cat.: HP40-117/2014E-PDF Pub.: 140118. Retrieved January 11, 2020.
- "What You Should Know for the 2018-2019 Influenza Season". Centers for Disease Control and Prevention (CDC). January 10, 2019. Retrieved February 5, 2020.

- "Fluzone High-Dose Quadrivalent". U.S. Food and Drug Administration (FDA). November 4, 2019. STN: BL 103914. Retrieved February 5, 2020.

- "FDA approves Fluzone High-Dose Quadrivalent (Influenza Vaccine) for adults 65 years of age and older". Sanofi (Press release). November 4, 2019. Retrieved February 5, 2020.
- Jit M, Newall AT, Beutels P (April 2013). "Key issues for estimating the impact and cost-effectiveness of seasonal influenza vaccination strategies". Human Vaccines & Immunotherapeutics. 9 (4): 834–40. doi:10.4161/hv.23637. PMC 3903903. PMID 23357859.
- Postma MJ, Baltussen RP, Palache AM, Wilschut JC (April 2006). "Further evidence for favorable cost-effectiveness of elderly influenza vaccination". Expert Review of Pharmacoeconomics & Outcomes Research. 6 (2): 215–27. doi:10.1586/14737167.6.2.215. PMID 20528557.
- Newall AT, Kelly H, Harsley S, Scuffham PA (2009). "Cost effectiveness of influenza vaccination in older adults: a critical review of economic evaluations for the 50- to 64-year age group". PharmacoEconomics. 27 (6): 439–50. doi:10.2165/00019053-200927060-00001. PMID 19640008.
- Newall AT, Viboud C, Wood JG (June 2010). "Influenza-attributable mortality in Australians aged more than 50 years: a comparison of different modelling approaches". Epidemiology and Infection. 138 (6): 836–42. doi:10.1017/S095026880999118X. PMID 19941685.
- Newall AT, Dehollain JP, Creighton P, Beutels P, Wood JG (August 2013). "Understanding the cost-effectiveness of influenza vaccination in children: methodological choices and seasonal variability". PharmacoEconomics. 31 (8): 693–702. doi:10.1007/s40273-013-0060-7. PMID 23645539.
- Newall AT, Scuffham PA (December 2011). "Uncertainty and variability in influenza cost-effectiveness models". Australian and New Zealand Journal of Public Health. 35 (6): 576, author reply 576–77. doi:10.1111/j.1753-6405.2011.00788.x. PMID 22151168.
- Gatwood J, Meltzer MI, Messonnier M, Ortega-Sanchez IR, Balkrishnan R, Prosser LA (January 2012). "Seasonal influenza vaccination of healthy working-age adults: a review of economic evaluations". Drugs. 72 (1): 35–48. doi:10.2165/11597310-000000000-00000. PMID 22191794.
- Newall AT, Jit M, Beutels P (August 2012). "Economic evaluations of childhood influenza vaccination: a critical review". PharmacoEconomics. 30 (8): 647–60. doi:10.2165/11599130-000000000-00000. PMID 22788257.
- Newall AT, Wood JG, Oudin N, MacIntyre CR (February 2010). "Cost-effectiveness of pharmaceutical-based pandemic influenza mitigation strategies". Emerging Infectious Diseases. 16 (2): 224–30. doi:10.3201/eid1602.090571. PMC 2957998. PMID 20113551.
- "Influenza Genome Sequencing Project – Overview". National Institutes of Health – National Institute of Allergy and Infectious Diseases. Archived from the original on June 27, 2011. Retrieved May 27, 2013.
- Lampos V, Yom-Tov E, Pebody R, Cox IJ (2015). "Assessing the impact of a health intervention via user-generated Internet content" (PDF). Data Mining and Knowledge Discovery. 29 (5): 1434–57. doi:10.1007/s10618-015-0427-9.
- Wagner M, Lampos V, Yom-Tov E, Pebody R, Cox IJ (December 2017). "Estimating the Population Impact of a New Pediatric Influenza Vaccination Program in England Using Social Media Content". Journal of Medical Internet Research. 19 (12): e416. doi:10.2196/jmir.8184. PMC 6257312. PMID 29269339.
- "Acute Respiratory Infections (Update September 2009)". World Health Organization (WHO). September 2009. Archived from the original on September 29, 2009. Retrieved September 16, 2009.
- "Tables on the Clinical trials of pandemic influenza prototype vaccines". World Health Organization (WHO). July 2009. Archived from the original on March 6, 2009. Retrieved September 21, 2009.
- "FDA Approves Vaccines for 2009 H1N1 Influenza Virus" (Press release). September 15, 2009. Archived from the original on October 15, 2009. Retrieved October 15, 2009.

- Keown, Alex (February 4, 2020). "FDA Approves Seqirus' Audenz as Vaccine Against Potential Flu Pandemic". BioSpace. Retrieved February 5, 2020.
- "Audenz". U.S. Food and Drug Administration (FDA). January 31, 2020. STN: 125692. Retrieved February 5, 2020.

- Cho A, Wrammert J (April 2016). "Implications of broadly neutralizing antibodies in the development of a universal influenza vaccine". Current Opinion in Virology. 17: 110–115. doi:10.1016/j.coviro.2016.03.002. PMC 4940123. PMID 27031684.
- Ocuno, Y.; et al. (May 1993). "A common neutralizing epitope conserved between the hemagglutinins of influenza A virus H1 and H2 strains". Journal of Virology. 67 (5): 2552–58. doi:10.1128/JVI.67.5.2552-2558.1993. PMC 237575. PMID 7682624.
- Soema PC, Kompier R, Amorij JP, Kersten GF (August 2015). "Current and next generation influenza vaccines: Formulation and production strategies". European Journal of Pharmaceutics and Biopharmaceutics. 94: 251–63. doi:10.1016/j.ejpb.2015.05.023. PMID 26047796.
- Deng L, Cho KJ, Fiers W, Saelens X (February 2015). "M2e-Based Universal Influenza A Vaccines". Vaccines. 3 (1): 105–36. doi:10.3390/vaccines3010105. PMC 4494237. PMID 26344949.
- Gottlieb T, Ben-Yedidia T (November 2014). "Epitope-based approaches to a universal influenza vaccine". Journal of Autoimmunity. 54: 15–20. doi:10.1016/j.jaut.2014.07.005. PMID 25172355.
- Toussi DN, Massari P (April 2014). "Immune Adjuvant Effect of Molecularly-defined Toll-Like Receptor Ligands". Vaccines. 2 (2): 323–53. doi:10.3390/vaccines2020323. PMC 4494261. PMID 26344622.
- Atsmon J, Caraco Y, Ziv-Sefer S, Shaikevich D, Abramov E, Volokhov I, Bruzil S, Haima KY, Gottlieb T, Ben-Yedidia T (October 2014). "Priming by a novel universal influenza vaccine (Multimeric-001)-a gateway for improving immune response in the elderly population". Vaccine. 32 (44): 5816–23. doi:10.1016/j.vaccine.2014.08.031. PMID 25173483.
- van Doorn E, Liu H, Ben-Yedidia T, Hassin S, Visontai I, Norley S, Frijlink HW, Hak E (March 2017). "Evaluating the immunogenicity and safety of a BiondVax-developed universal influenza vaccine (Multimeric-001) either as a standalone vaccine or as a primer to H5N1 influenza vaccine: Phase IIb study protocol". Medicine (Baltimore). 96 (11): e6339. doi:10.1097/MD.0000000000006339. PMC 5369918. PMID 28296763.
- Clinical trial number NCT03058692 for "Two Doses of Multimeric-001 (M-001) Followed by Influenza Vaccine" at ClinicalTrials.gov
- Clinical trial number NCT03450915 for "A Pivotal Trial to Assess the Safety and Clinical Efficacy of the M-001 as a Standalone Universal Flu Vaccine" at ClinicalTrials.gov
- "NIH begins first-in-human trial of a universal influenza vaccine candidate". National Institutes of Health (NIH) (Press release). April 3, 2019. Archived from the original on November 12, 2019. Retrieved November 12, 2019.
- Clinical trial number NCT03814720 for "Dose, Safety, Tolerability and Immunogenicity of an Influenza H1 Stabilized Stem Ferritin Vaccine, VRCFLUNPF099-00-VP, in Healthy Adults" at ClinicalTrials.gov
- Sederstrom, Jill (July 19, 2019). "Researchers Exploring Oral Flu Vaccine and Treatment Options". Drug Topics. Retrieved January 27, 2020.
- Fast, Patricia E; Cox, Josephine H (September 1, 2015). "An influenza vaccine pill-can we swallow it?". The Lancet Infectious Diseases. 15 (9): 1051–6, discussion 1056–57. doi:10.1016/s1473-3099(15)00252-2. PMID 2633331. Retrieved January 27, 2020.
- Liebowitz, David; Gottlieb, Keith; Kolhatkar, Nikita S; Garg, Shaily J; Asher, Jason M; Nazareno, Jonathan (January 21, 2020). "Efficacy, immunogenicity, and safety of an oral influenza vaccine: a placebo-controlled and active-controlled phase 2 human challenge study". The Lancet Infectious Diseases. Elsevier. 20 (4): 435–444. doi:10.1016/S1473-3099(19)30584-5. PMID 31978354. Retrieved January 27, 2020.
- Influenza Report (online book) Archived May 10, 2016, at the Wayback Machine chapter Avian Influenza by Timm C. Harder and Ortrud Werner
- equiflunet_vaccines Archived January 10, 2006, at the Wayback Machine
- "UAE Equestrian & Racing Federation". March 22, 2005. Archived from the original on March 22, 2005.
- "FEI guidelines" (PDF). Archived (PDF) from the original on October 26, 2007.
- McNeil DG Jr (May 2, 2006). "Turning to Chickens in Fight With Bird Flu". The New York Times. Archived from the original on November 14, 2012.
- "Bird flu warning over partial protection of flocks". August 16, 2006. Archived from the original on September 26, 2007.
- "FAO Recommendations on the Prevention, Control and Eradication of Highly Pathogenic Avian Influenza (HPAI) in Asia" (PDF). Archived (PDF) from the original on February 2, 2010. Retrieved September 16, 2009.
- Yen HL, Peiris JS (May 2012). "Virology: bird flu in mammals". Nature. 486 (7403): 332–33. Bibcode:2012Natur.486..332Y. doi:10.1038/nature11192. PMID 22722190.
- "Swine Flu Virus Turns Endemic". September 15, 2007. Archived from the original on April 29, 2009.
- "Custom Vaccines: Swine". Archived from the original on April 30, 2009.
- Karaca K, Dubovi EJ, Siger L, Robles A, Audonnet JC, Jiansheng Y, et al. (February 2007). "Evaluation of the ability of canarypox-vectored equine influenza virus vaccines to induce humoral immune responses against canine influenza viruses in dogs". American Journal of Veterinary Research. 68 (2): 208–12. doi:10.2460/ajvr.68.2.208. PMID 17269888.
Further reading
- World Health Organization (October 2017). The immunological basis for immunization series: module 23: influenza vaccines. World Health Organization (WHO). hdl:10665/259211. ISBN 978-9241513050.
- Ramsay M (ed.). "Chapter 19: Influenza". Immunisation against infectious disease. Public Health England.
- Hamborsky J, Kroger A, Wolfe S, eds. (2015). "Chapter 12: Influenza". Epidemiology and Prevention of Vaccine-Preventable Diseases (13th ed.). Washington D.C.: U.S. Centers for Disease Control and Prevention (CDC). ISBN 978-0990449119.
- Budd A, Blanton L, Grohskopf L, Campbell A, Dugan V, Wentworth DE, et al. (March 29, 2019). "Chapter 6: Influenza". In Roush SW, Baldy LM, Hall MA (eds.). Manual for the surveillance of vaccine-preventable diseases. Atlanta GA: U.S. Centers for Disease Control and Prevention (CDC).
- National Advisory Committee on Immunization (May 2020). "Canadian Immunization Guide Chapter on Influenza and Statement on Seasonal Influenza Vaccine for 2020–2021" (PDF). Public Health Agency of Canada. Cat.: HP37-25F-PDF; Pub.: 200003. Lay summary.
- National Advisory Committee on Immunization (NACI) (May 2018). "NACI literature review on the comparative effectiveness and immunogenicity of subunit and split virus inactivated influenza vaccines in adults 65 years of age and older" (PDF). Government of Canada. ISBN 9780660264387. Cat.: HP40-213/2018E-PDF; Pub.: 180039. Lay summary.
External links
- Inactivated Influenza Vaccine Information Statement, US Centers for Disease Control and Prevention (CDC)
- Live, Intranasal Influenza Vaccine Information Statement, US Centers for Disease Control and Prevention (CDC)
- Seasonal Influenza (Flu) Vaccination and Preventable Disease, US Centers for Disease Control and Prevention (CDC)
- Misconceptions about Seasonal Flu and Flu Vaccines, US Centers for Disease Control and Prevention (CDC)
- Influenza Vaccines at the US National Library of Medicine Medical Subject Headings (MeSH)
- "Influenza Vaccine". Drug Information Portal. U.S. National Library of Medicine.