CSL Limited
CSL Limited is a global specialty biotechnology company that researches, develops, manufactures, and markets products to treat and prevent serious human medical conditions. CSL's product areas include blood plasma derivatives, vaccines, antivenom, and cell culture reagents used in various medical and genetic research and manufacturing applications.[2]
 | |
| Public | |
| Traded as | ASX: CSL |
| ISIN | AU000000CSL8 |
| Industry | Biotechnology |
| Founded | 1916 (Federal government department), 1994 (privatised) |
| Headquarters | Parkville, Melbourne, Victoria, Australia[1] |
Key people | Paul Perreault (CEO) |
| Products | blood plasma, vaccines, antivenom, other laboratory and medical products |
| Revenue | |
Number of employees | 25,000 person (2019)[1] |
| Divisions |
|
| Website | www |
History
CSL was founded in 1916 as the Commonwealth Serum Laboratories, an Australian government body focused on vaccine manufacture. Under the first director, William Penfold,[3] CSL commenced operation in the vacant Walter and Eliza Hall Institute building at the Melbourne Hospital in 1918, before moving to its purpose-built Parkville premises in the following year. After ongoing disputes with the Commonwealth Department of Health and its director, (John) Howard Cumpston, Penfold resigned in 1927 and was replaced by Frederic Morgan. Soon after Morgan's appointment, CSL was drawn into a serious public health disaster when a batch of its diphtheria toxin-antitoxin was implicated in the deaths of twelve children in what became known as the 'Bundaberg tragedy' of 1928. Although CSL's manufacturing processes were absolved, its labelling procedures were seen to be in error, leading to an enduring focus on the highest standards across the facility's production.[4]
In 1928, CSL also became involved in antivenene (antivenom) manufacture in conjunction with the snake venom research undertaken by Charles Kellaway at the Hall Institute. This led to the successful clinical testing of antivenene against tiger snake Notechis scutatus bite in 1930, and its commercial release in 1931. In 1934, the research on snake venoms was transferred from the Hall Institute to CSL under the direction of former snake showman, Tom 'Pambo' Eades. This represented the initiation of research at the laboratories – an outcome its directors had been seeking for over a decade. The relationship with the Hall Institute continued until World War II, particularly via joint projects on viral diseases including polio and influenza coordinated by Frank Macfarlane Burnet and Esmond 'Bill' Keogh. Keogh played an important role in the establishment of penicillin production at CSL in 1944 – a critical wartime achievement.[5]
The operation commenced plasma fractionation in 1952. Thereafter the range of antivenoms increased, including those against other snake species such as death adder (Acanthophis antarcticus) and the taipan (Oxyuranus scutellatus), plus spiders including the redback (Latrodectus hasselti) and – after much difficulty – the Sydney funnel-web (Atrax robustus). Much of this work, including the introduction in 1962 of a polyvalent antivenom against all of the major terrestrial Australian snakes, occurred under the direction of Saul Wiener, while from 1966 until the mid-1990s, venom research was coordinated by the eccentric but dedicated Struan Sutherland, who in 1979 released new guidelines for snakebite first aid,[6] and a new test for snakebites that would identify which snake had envenomated the victim.[7]
Other major achievements of CSL include:[8]
- early production of insulin for treatment of Australian diabetics (1923)
- development of a tetanus vaccine (1938)
- development of a combined vaccine for diphtheria, tetanus and whooping cough (1953)
- rapid adoption and production of a polio vaccine (1956)
- development of a multi-purpose animal vaccine covering pulpy kidney (enterotoxemia), tetanus, black disease, malignant oedema and blackleg (1961)
- production of Rhesus (D) immunoglobulin to prevent haemolytic disease in newborns due to Rh factor incompatibility (1966–67)
- pioneering heat treatment to protect blood and plasma products from infection with HIV (1983)
- collaboration on development of the world's first human papillomavirus vaccine, Gardasil, building on the pioneering work by Professor Ian Frazer (1994-2005).[9]
In 1994, the Commonwealth facility was privatised as CSL Ltd. In 2000 CSL doubled its size through the purchase of a Swiss plasma company, the Bern-based ZLB Bioplasma AG. In 2004, during a period of plasma oversupply, the company expanded again with the purchase of the German medical company Aventis Behring. The company was the 2nd Australian public company to have reached a share price of over $100 per share.
In 2011, the company received the Minister's Award for Outstanding Equal Employment Opportunities Initiative for their Thinking Kids Children's Centre.[10]
In October 2014, Novartis announced its intention to sell its influenza vaccine business, including its development pipeline, to CSL for $275 million. CSL merged it into its BioCSL operation.[11] In November 2015, BioCSL rebranded the combined business with Novartis Influenza Vaccines as Seqirus [Sek-eer-us] creating the world's second largest influenza vaccine company.[12] Completed in 2018, Seqirus's Holly Spring, NC, plant was funded with $59 million from the U.S. government.[13]
Locations
The company's headquarters remain in Parkville, Victoria, an inner suburb of Melbourne. CSL Behring is headquartered in King of Prussia, USA and it has manufacturing operations and R&D laboratories in the Swiss city of Bern, in Marburg in Germany, and Kankakee, USA.
Seqirus has its headquarters in Maidenhead and has production facilities in Holly Springs, USA, Liverpool, UK, and Parkville, Victoria
Ownership
CSL is a public company and its stock is traded on the Australian Securities Exchange under the stock code CSL. The company completed an Initial Public Offering in June 1994 at A$2.30 per share. CSL stock is part of the S&P/ASX 20 Index.[14]
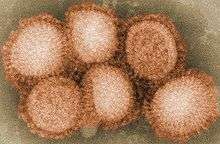
Vaccine for A/H1N1 2009 Pandemic
| Influenza (Flu) |
|---|
 |
|
CSL's vaccine for Swine Flu, the world's first, was approved in September 2009 for use by people aged 10 and over.[15] The federal government ordered 21 million doses of vaccine for Australians. Further doses were made for customers in Singapore and USA. 2009 A/H1N1 . The Australian government intended to use the CSL Vaccine in one of the largest national vaccine programs in the country's history.
Divisions
CSL Limited's products can be separated by company division. Some of the key products produced by each division, have included:
Seqirus (bioCSL)
- Afluria (influenza vaccine) -- Argentina, Peru, South Africa, Spain, US[16]
- Afluria Quadrivalent (influenza vaccine) -- Australia, Canada, New Zealand, US[16]
- Agrippal (influenza vaccine) -- Argentina, Austria, Brazil, Canada, Chile, Colombia, Germany, Italy, Mexico, Spain, Thailand[16]
- Audenz (influenza A (H5N1) vaccine) -- US[16]
- Fluad (influenza vaccine) -- Argentina, Austria, Australia, Brazil, Canada, Denmark, Germany, Italy, Spain, Switzerland, UK, US[16]
- Chiromas -- Spain[16]
- Fluad Pediatric (influenza vaccine) -- Canada[16]
- Flucelvax Quadrivalent (influenza vaccine) -- Germany, Italy, Spain, UK, US[16]
- Q-VAX (Coxiella burnetii vaccine) -- Australia[17]
- Rapivab (peramivir) -- Australia, US[16]
Antivenoms: (Australia)[17]
- Black snake
- Box jellyfish
- Brown snake
- Death adder
- Funnel web spider
- Polyvalent snake antivenom
- Redback spider
- Sea snake
- Stonefish
- Taipan
- Tiger snake
- Snake Venom Detection Kit
CSL Behring (Australia)[18]
- Albumex (serum albumin)
- Biostate (Factor VIII)
- Carimune, which is immunoglobulin for intravenous administration (IGIV)
- CMV Immunoglobulin-VF (cytomegalovirus immunoglobulin)
- Helixate, which is recombinant Antihemophilic Factor, a blood-clotting factor for the treatment of haemophilia
- Hepatitis B immunoglobulin
- human immunoglobulin – Intragam P, Normal, Rh(D) Immunoglobulin-VF, Sandoglobulin,
- MonoFIX-VF (Factor IX)
- Prothrombinex-HT (prothrombin complex)
- Rhophylac (Rh(D) immunoglobulin G)
- Tetanus Immunoglobulin-VF
- Thrombotrol-VF (antithrombin III)
- Vivaglobin, sub-cutaneous human immune globulin indicated for the treatment of primary immunodeficiency. This product gained FDA approval in January 2006.
- Von Willebrand factor
- Zoster Immunoglobulin-VF (varicella zoster immunoglobulin)
CSL Behring
- Beriglobin P, human hepatitis A immunoglobulin, liquid 16% solution for intramuscular injection
- Berirab P, human rabies immunoglobulin, liquid 16% solution for intramuscular injection
- Carimune NF, Sandoglobulin, Sanglopor human normal immunoglobulin, freeze-dried formulations for intravenous administration
- Cytogam, human cytomegalovirus immunoglobulin. Liquid immunoglobulin containing a standardized amount of antibody to cytomegalovirus.
- Hepatitis B Immunoglobulin P Behring, human hepatitis B immunoglobulin, liquid 16% solution for intramuscular injection
- Hizentra,Human normal immunoglobulin. Liquid 20% immunoglobulin solution, ready-to-use for subcutaneous administration
- Privigen, human polyvalent immunoglobulin, liquid 10% solution for intravenous injection
- Rhesogamma P, human anti-D immunoglobulin. Prefilled syringes of highly purified anti-Rhesus factor D IgG for intravenous administration and intramuscular injection.
- Rhophylac human anti-D immunoglobulin. Prefilled syringes of highly purified anti-Rhesus factor D IgG for intravenous administration and intramuscular injection.
- Sandoglobulin NF Liquid, Redimune, Redimune NF Liquid, human normal immunoglobulin, liquid 12% solution for intravenous administration
- Tetagam P, human tetanus immunoglobulin, liquid 16% solution for intramuscular injection
- Varicellon P, human varicella immunoglobulin, liquid 16% solution for intramuscular injection
- Vivaglobin, human normal immunoglobulin, liquid 16% solution for subcutaneous administration
Coagulation/Bleeding Disorders:
- Beriate, freeze-dried human coagulation factor VIII concentrate
- Berinin P, freeze-dried human coagulation factor IX concentrate
- Factor X P Behring, a freeze-dried human coagulation factor IX and factor X concentrate
- Fibrogammin P, Cluvot and Corifact, freeze-dried human coagulation factor XIII concentrate
- Helixate FS and Helixate NexGen, freeze-dried recombinant coagulation factor VIII
- Humate-P and Haemate P, freeze-dried human coagulation factor VIII: C and von Willebrand factor concentrate
- Monoclate P, a freeze-dried monoclonal antibody purified human coagulation factor VIII concentrate
- Mononine, a freeze-dried human coagulation factor IX that has been purified using monoclonal antibodies
- Stimate, a synthetic desmopressin acetate nasal spray
- Octostim, a synthetic desmopressin acetate nasal spray
Pulmonary:
- Zemaira, Respreeza freeze-dried Human Alpha1-proteinase inhibitor (A1-PI)
Critical Care:
- AlbuRx, Alburex, Albumeon, Human Albumin Behring, Albuminar 25, human albumin solution (5%, 20% or 25% human albumin solutions)
- Berinert P, freeze-dried human C1-esterase inhibitor (C1-INH) concentrate
- Beriplex P/N, freeze-dried human prothrombin complex concentrate
- Haemocomplettan P, RiaSTAP, freeze-dried human fibrinogen (factor I) concentrate
- Kybernin P, freeze-dried human antithrombin III concentrate
- Streptase, freeze-dried streptokinase
Wound Healing:
- Beriplast P Combi-Set, fibrin sealant kit, freeze-dried fibrin sealant for topical application
- Fibrogammin P, freeze-dried human coagulation factor XIII concentrate
- TachoComb, fibrin sealant fleece-type, fleece-type collagen preparations coated with fibrin glue components
Product availability varies from country to country, depending on registration status.
See also
- Australian Red Cross Blood Service
References
- "Annual Report 2019" (PDF). CSL Limited. 2019.
- "CSL LTD (CSL:ASX): Stock Quote & Company Profile - Businessweek". businessweek. Archived from the original on 18 January 2013. Retrieved 13 August 2012.
- Robin, A. De Q. Penfold, William James (1875–1941). Canberra: National Centre of Biography, Australian National University.
- "BUNDABERG TRAGEDY, Daily Examiner". 14 June 1928. p. 3 – via Trove.
- Gardiner, Lyndsay; Serle, Geoffrey (2000). "Keogh, Esmond Venner (Bill) (1895–1970)". Australian Dictionary of Biography. National Centre of Biography, Australian National University. 15.
- "SAFER FIRST AID, Papua New Guinea Post-Courier". 18 April 1979. p. 11 – via Trove.
- "New test for snake bites, The Canberra Times". 30 October 1979. p. 14 – via Trove.
- Tasker, Sarah-Jane (23 April 2016). "Blood, sweat and tears of the CSL century". The Australian.
- "A global solution to reducing cervical cancer" (PDF). Uniquest commercialisation stories. The University of Queensland. Retrieved 29 April 2016.
- "CSL wins equal opportunity award for onsite childcare centre". CSL Newsroom. CSL. Retrieved 25 October 2016.
- Phillipidis, Alex (27 October 2014). "Novartis Selling Flu Vaccine Business to CSL for $275M". Genetic Engineering & Biotechnology News. Retrieved 22 May 2020.
- Sequirus Commonwealth Serum Laboratories
- Willman, David (15 March 2020). "Federal vaccine development sites ill-suited to counter covid-19 epidemic". Washington Post. Retrieved 15 March 2020.
- "CSL Limited". Australian Securities Exchange. Retrieved 7 February 2020.
- "Panvax H1N1 Approval For Registration For Use in Australia by Therapeutic Goods Administration". Melbourne, Australia: CSL Limited. 18 September 2009. Archived from the original on 18 September 2009. Retrieved 26 September 2009.
CSL Biotherapies, a subsidiary of CSL Limited, Australia's leading biopharmaceutical company, can today confirm that its vaccine against the pandemic (H1N1) 2009 influenza or 'swine flu' has been approved registration for use in people aged 10 years and over.
- "Products". Seqirus. 6 December 2018. Retrieved 5 February 2020.
- "Products". Seqirus. Australia. Retrieved 9 February 2020.
- "Products". cslbehring.com.au. Retrieved 7 February 2020.
- Global product portfolio CSL Behring, 3 November 2010
Sources
- AH Brogan, Committed to Saving Lives: a History of the Commonwealth Serum Laboratories (Melbourne: Hyland House, 1990).
- Dando McCredie, The Fight Against Disease and CSL's Seventy Year Contribution (Richmond: Dando McCredie, c.1986).
- FG Morgan, 'The Commonwealth Serum Laboratories and their work', Collected Proceedings of the Society of Chemical Industry of Victoria, XXXV (1935), 1015–31.
- WJ Penfold, 'The Commonwealth Serum Laboratories', Medical Journal of Australia, 1 (14 April 1923), 396–400.
- Struan K Sutherland, A Venomous Life: the Autobiography of Professor Struan Sutherland (Melbourne: Hyland House, 1998).