Group B streptococcal infection
Group B streptococcal infection, also known as Group B streptococcal disease or just Group B strep,[1] is the infection caused by the bacterium Streptococcus agalactiae (S. agalactiae) (also known as group B streptococcus or GBS). GBS infection can cause serious illness and sometimes death, especially in newborns, the elderly, and people with compromised immune systems.
| Group B Streptococcal infection | |
|---|---|
| Other names | Group B streptococcal disease |
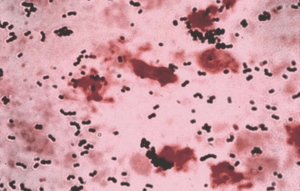 | |
| Streptococcus agalactiae- Gram stain | |
| Specialty | Pediatrics |
As other virulent bacteria, GBS harbours an important number of virulence factors,[2] the most important are the capsular polysaccharide (rich in sialic acid), and a pore-forming toxin, β-haemolysin.[3][4] The GBS capsule is probably the key virulence factor because it helps GBS escape from the host defence mechanisms interfering with phagocytic killing of GBS by human phagocytes.[5][3] The GBS β-haemolysin is considered almost identical to the GBS pigment (granadaene).[6][7][8][9]
GBS was recognized as a pathogen in cattle by Edmond Nocard and Mollereau in the late 1880s. It can cause bovine mastitis (inflammation of the udder) in dairy cows. The species name "agalactiae" meaning "no milk", alludes to this.[10]
Its significance as a human pathogen was first described in 1938, when Fry reported three fatal cases of puerperal infections caused by GBS.[11] In the early 1960s, GBS was recognized as a main cause of infections in newborns.[12]
In general, GBS is a harmless commensal bacterium being part of the human microbiota colonizing the gastrointestinal and genitourinary tracts of up to 30% of healthy human adults (asymptomatic carriers).[13][14][5]
Laboratory identification
As mentioned, S. agalactiae is a Gram-positive coccus with a tendency to form chains, beta-haemolytic, catalase-negative, and facultative anaerobe. GBS grows readily on blood agar plates as microbial colonies surrounded by a narrow zone of β-haemolysis. GBS is characterized by the presence in the cell wall of the group B antigen of the Lancefield classification (Lancefield grouping) that can be detected directly in intact bacteria using latex agglutination tests.[15][16] The CAMP test is also another important test for the identification of GBS. The CAMP factor acts synergistically with the staphylococcal β-haemolysin inducing enhanced haemolysis of sheep or bovine erythrocytes.[15]
GBS is also able to hydrolyse hippurate, and this test can also be used to identify GBS. Haemolytic GBS strains produce an orange-brick-red nonisoprenoid polyene pigment (ornythinrhamnododecaene) (granadaene) when cultivated on granada medium that allows its straightforward identification.[17]
Identification of GBS could also be carried out easily using modern methods as matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry.[18][19]
Additionally GBS colonies can be tentatively identified after their appearance in chromogenic agar media.[16][20][21] Nevertheless GBS-like colonies that develop in chromogenic media should be confirmed as GBS using additional reliable tests (e.g.latex agglutination or the CAMP test) to avoid potential mis-identification.[16] A summary of the laboratory techniques for GBS identification is depicted in Ref 18.[16]
Colonization versus infection
GBS is found in the gastrointestinal and genitourinary tract of humans and is normal component of the intestinal and vaginal microbiota in some women.[22] In different studies, GBS vaginal colonization rate ranges from 4 to 36%, with most studies reporting rates over 20%. Vaginal or rectal colonization may be intermittent, transitory, or persistent.[22] These variations in the reported prevalence of asymptomatic (presenting no symptoms of disease) colonization could be related to the different detection methods used, and differences in populations studied.[23][24][20]
Though GBS is an asymptomatic and harmless colonizer of the gastrointestinal human tract in up to 30% of otherwise healthy adults, including pregnant women,[5][23] this opportunistic harmless bacterium can, in some circumstances, cause severe invasive infections.[14]
Pregnancy
Though GBS colonization is asymptomatic and, in general, does not cause problems, it can sometimes cause serious illness for the mother and the baby during gestation and after delivery. GBS infections in the mother can cause chorioamnionitis (intra-amniotic infection or severe infection of the placental tissues) infrequently, postpartum infections (after birth) and it had been related with prematurity and fetal death.[25] GBS urinary tract infections may induce labour in pregnant women and cause premature delivery (preterm birth) and miscarriage.[5][26]
Newborns
In the western world, GBS (in the absence of effective prevention measures) is the main cause of bacterial infections in newborns, such as sepsis, pneumonia, and meningitis, which can lead to death or long-term after effects.[5][27]
GBS infections in newborns are separated into two clinical types, early-onset disease (GBS-EOD) and late-onset disease (GBS-LOD). GBS-EOD manifests from 0 to 7 living days in the newborn, most of the cases of EOD being apparent within 24 h from birth. GBS-LOD starts between 7 and 90 days after birth.[5][20]
The most common clinical syndromes of GBS-EOD are sepsis without apparent location, pneumonia, and less frequently meningitis. Bacteremia without a focus occurs in 80-85%, pneumonia in 10-15%, and meningitis in 5-10% of cases. The initial clinical findings are respiratory signs in more than 80% of cases. Neonates with meningitis often have an initial clinical presentation identical to presentation in those without meningeal affectation. An exam of the cerebrospinal fluid is often necessary to rule out meningitis.[5][28][29]
Colonization with GBS during labour is the primary risk factor for the development of GBS-EOD. GBS-EOD is acquired vertically (vertical transmission), through exposure of the fetus or the baby to GBS from the vagina of a colonized woman, either in utero (because of ascending infection) or during birth, after rupture of membranes. Infants can also be infected during passage through the birth canal, nevertheless, newborns who acquire GBS through this route can only become colonized, and these colonized infants usually do not develop GBS-EOD.
Roughly 50% of newborns of GBS colonized mothers are also GBS colonized and (without prevention measures) 1-2% of these newborns will develop GBS-EOD.[30]
Though maternal GBS colonization is the key determinant for GBS-EOD, other factors also increase the risk. These factors are:[5][20]
- Onset of labour before 37 weeks of gestation (premature birth)
- Prolonged rupture of membranes (longer duration of membrane rupture) (≥18 h before delivery)
- GBS bacteriuria during pregnancy
- Intrapartum (during childbirth) fever (>38 °C, >100.4 °F)
- Amniotic infections (chorioamnionitis)
- Young maternal age
- Maternal HIV-infection[31]
Nevertheless, most babies who develop GBS-EOD are born to colonized mothers without any of these risk factors.[20] Heavy GBS vaginal colonization is also associated with a higher risk for GBS-EOD. Women who had one of these risk factors but who are not GBS colonized at labour are at low risk for GBS-EOD compared to women who were colonized prenatally, but had none of the aforementioned risk factors.[30]
Presence of low levels of anticapsular antibodies against GBS in the mother are also of great importance for the development of GBS-EOD.[32][33] Because of that, a previous sibling with GBS-EOD is also an important risk factor for the development of the infection in subsequent deliveries, probably reflecting the lack of protective antibodies in the mother. [20]
Overall, the case fatality rates from GBS-EOD have declined, from 50% observed in studies from the 1970s to between 2 and 10% in recent years, mainly as a consequence of improvements in therapy and management. Fatal neonatal infections by GBS are more frequent among premature infants. [5] [20] [34]
GBS-LOD affects infants from 7 days to 3 months of age and has a lower case fatality rate (1%-6%) than GBS-EOD. Clinical syndromes of GBS-EOD are bacteremia without a focus (65%), meningitis (25%), cellulitis, osteoarthritis, and pneumonia. Prematurity has been reported to be the main risk factor. Each week of decreasing gestation increases the risk by a factor of 1.34 for developing GBS-LOD. [35]
GBS-LOD is not acquired through vertical transmission during delivery; it can be acquired later from the mother from breast milk or from environmental and community sources. GBS-LOD commonly shows nonspecific signs, and diagnosis should be made obtaining blood cultures in febrile newborns. S.agalactiae neonatal meningitis does not present with the hallmark sign of adult meningitis, a stiff neck; rather, it presents with nonspecific symptoms, such as fever, vomiting and irritability, and can consequently lead to a late diagnosis. Hearing loss and mental impairment can be a long-term consequence of GBS meningitis.[5][27]
Prevention of neonatal infection
Currently, the only reliable way to prevent GBS-EOD is intrapartum antibiotic prophylaxis (IAP) - administration of intravenous (IV) antibiotics during delivery. Intravenous penicillin or ampicillin given at the onset of labour and then again every four hours until delivery to GBS colonized women have been proven to be very effective at preventing vertical transmission of GBS from mother to baby and GBS-EOD (penicillin G, 5 million units IV initial dose, then 3 million units[22] every 4 hours until delivery or ampicillin, 2 g IV initial dose, then 1 g IV every 4 hours until delivery).[5][20][22]
Penicillin-allergic women without a history of anaphylaxis (angioedema, respiratory distress, or urticaria) following administration of a penicillin or a cephalosporin (low risk of anaphylaxis) could receive cefazolin (2 g IV initial dose, then 1 g IV every 8 hours until delivery) instead of penicillin or ampicillin.[20] Clindamycin (900 mg IV every 8 hours until delivery), Erythromycin is not recommended today because the high proportion of GBS resistance to erythromycin (up to 44.8%),[20][22]
Neither oral or intramuscular antibiotics are effective in reducing the risk GBS EOD.[22]
Antibiotic susceptibility testing of GBS isolates is crucial for appropriate antibiotic selection for IAP in penicillin-allergic women, because resistance to clindamycin, the most common agent used (in penicillin-allergic women), is increasing among GBS isolates. Appropriate methodologies for testing are important, because resistance to clyndamicin (antimicrobial resistance) can occur in some GBS strains that appear susceptible (antibiotic sensitivity) in certain susceptibility tests.[20]
For women who are at risk of anaphylaxis after exposure to penicillin, the laboratory requisitions should indicate clearly the presence of penicillin allergy to ensure that the laboratory is aware for the need of testing GBS isolates for clindamycin susceptibility. Vancomycin (20 mg/Kg every 8 hours until delivery)[22] is used to prevent GBS-EOD in infants born to penicillin-allergic mothers.[20][22]
If appropriate IAP in GBS colonized women starts at least 2 hours before the delivery, the risk of neonatal infection is also somehow reduced.[36][37][38]
True penicillin allergy is rare with an estimated frequency of anaphylaxis of one to five episodes per 10,000 cases of penicillin therapy.[39] Penicillin administered to a woman with no history of β-lactam allergy has a risk of anaphylaxis of 0.04 to 4 per 100,000. Maternal anaphylaxis associated with GBS IAP occurs, but any morbidity associated with anaphylaxis is offset greatly by reductions in the incidence of GBS-EOD. [20]
IAPs have been considered to be associated with the emergence of resistant bacterial strains and with an increase in the incidence of early-onset infections caused by other pathogens, mainly Gram-negative bacteria such as Escherichia coli. Nevertheless, most studies have not found an increased rate of non-GBS early-onset sepsis related to the widespread use of IAP. [20][40][41][42]
Other strategies to prevent GBS-EOD have been studied, and chlorhexidine intrapartum vaginal cleansing has been proposed to help preventing GBS-EOD, nevertheless no evidence has been shown for the effectiveness of this approach.[20][22][43][44]
Identifying candidates to receive IAP
Two ways are used to select female candidates to IAP: the culture-based screening approach and the risk-based approach.[45] The culture-based screening approach identifies candidates using lower vaginal and rectal cultures obtained between 35 and 37 weeks of gestation (or 36-37[22]), and IAP is administered to all GBS colonized women. The risk-based strategy identifies candidates to receive IAP by the aforementioned risk factors known to increase the probability of GBS-EOD without considering if the mother is or is not a GBS carrier.[5][20][46]
IAP is also recommended for women with intrapartum risk factors if their GBS carrier status is not known at the time of delivery, and for women with GBS bacteriuria during their pregnancy, and for women who have had an infant with GBS-EOD previously. The risk-based approach is, in general, less effective than the culture-based approach, [47] because in most cases, GBS-EOD develops among newborns who have been born to mothers without risk factors.[20][30][48]
IAP is not required for women undergoing planned caesarean section in the absence of labour and with intact membranes, irrespective of the carriage of GBS.[20][22]
Routine screening of pregnant women is performed in most developed countries such as the United States, France, Spain, Belgium, Canada, and Australia, and data have shown falling incidences of GBS-EOD following the introduction of screening-based measures to prevent GBS-EOD.[24][48] [49]
The risk-based strategy is advocated, among other counties, in the United Kingdom, the Netherlands, New Zealand, and Argentina.[24]
The issue of cost-effectiveness of both strategies for identifying candidates for IAP is less clear-cut, and some studies have indicated that testing low risk women, plus IAP administered to high-risk women, and to those found to carry GBS is more cost-effective than the current UK practice.[50] Other evaluations have also found the culture-based approach to be more cost-effective than the risk-based approach for the prevention of GBS-EOD.[51][52]
Testing pregnant women to detect GBS carriers has also been proposed, and giving IAP to those carrying GBS and to high-risk women, is significantly more cost-effective than the use of the risk-factor approach. One research paper calculated an expected net benefit to the UK government of such an approach of around £37million a year, compared with the current RCOG approach.[50][51]
It has been reported that IAP does to not prevent all cases of GBS-EOD; its efficacy is estimated at 80%. The risk-based prevention strategy does not prevent about 33% of cases with no risk factors.[53]
Up to 90% of cases of GBS-EOD would be preventable if IAP were offered to all GBS carriers identified by universal screening late in pregnancy, plus to the mothers in higher risk situations.[54]
Where insufficient intravenous antibiotics are given before delivery, the baby may be given antibiotics immediately after birth, although evidence is inconclusive as to whether this practice is effective or not.[20][55][56][57]
Home births and water birth
Home births are becoming increasingly popular in the UK. Recommendations for preventing GBS infections in newborns are the same for home births as for hospital births. Around 25% of women having home births probably carry GBS in their vaginas at delivery without knowing, and it could be difficult to follow correctly the recommendations of IAP and to deal with the risk of a severe allergic reaction to the antibiotics outside of a hospital setting.[58]
The RCOG and the ACOG guidelines suggests that birth in a pool is not contraindicated for GBS carriers who have been offered the appropriate IAP if no other contraindications to water immersion are present[22][59]
Screening for colonization
Approximately 10–30% of women are colonized with GBS during pregnancy. Nevertheless, during pregnancy, colonization can be temporary, intermittent, or continual.[20] Because the GBS colonization status of women can change during pregnancy, only cultures carried out ≤5 weeks before delivery predict quite accurately the GBS carrier status at delivery.[60] In contrast, if the prenatal culture is carried out more than 5 weeks before delivery, it is unreliable for accurately predicting the GBS carrier status at delivery. Because of that, testing for GBS colonization in pregnant women is recommended by the CDC at 35–37 weeks of gestation.[20][61] It is important to note that the ACOG now recommends performing universal GBS screening between 36 and 37 weeks of gestation. This new recommendation provides a 5-week window for valid culture results that includes births that occur up to a gestational age of at least 41 weeks.[22]
The clinical samples recommended for culture of GBS are swabs collected from the lower vagina and rectum through the external anal sphincter. The sample should be collected swabbing the lower vagina (vaginal introitus) followed by the rectum (i.e., inserting the swab through the anal sphincter) using the same swab or two different swabs. Cervical, perianal, perirectal, or perineal specimens are not acceptable, and a speculum should not be used for sample collection.[20] Samples can be taken by healthcare professionals, or by the mother herself with appropriate instruction.[62][63][64]
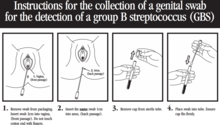
Following the recommendations of the CDC, these swabs should be placed into a non-nutritive transport medium. When feasible, specimens should be refrigerated and sent to the laboratory as soon as possible.[20] Appropriate transport systems are commercially available, and in these transport media, GBS can remain viable for several days at room temperature. However, the recovery of GBS declines over one to four days, especially at elevated temperatures, which can lead to false-negative results.[20][65]
Culture methods
Samples (vaginal, rectal, or vaginorectal swabs) should be inoculated into a selective enrichment broth, (Todd Hewitt broth with selective antibiotics, enrichment culture). This involves growing the samples in an enriched medium to improve the viability of the GBS and simultaneously impairing the growth of other naturally occurring bacteria. After incubation (18–24 hours, 35-37 °C), the enrichment broth is subcultured to blood agar plates and GBS-like colonies are identified by the CAMP test or using latex agglutination with GBS antiserum.[20][66] In the UK, this is the method described by the Public Health England’s UK Standards for Microbiology Investigations[67]
After incubation, the enrichment broth can also be subcultured to granada medium agar where GBS grows as pinkish-red colonies[16][17][66][68] [69] or to chromogenic agars, where GBS grows as coloured colonies.[20] Nevertheless GBS-like colonies that develop in chromogenic media should be confirmed as GBS using additional reliable tests to avoid mis-identification.[16]
Inoculating directly the vaginal and rectal swabs or the vaginorectal swab in a plate of an appropriate culture medium (blood agar, granada medium or chromogenic media) is also possible. However, this method (bypassing the selective enrichment broth step) can lead to some false-negative results, and this approach should be taken only in addition to, and not instead of, inoculation into selective broth.[20]
Today, in the UK, the detection of GBS colonization using the enrichment broth technique is not offered from most laboratories serving the NHS. However, the implementation of this test seems to be a viable option. At present, culture for GBS (using enriched culture medium) at 35–37 weeks to define an at-risk group of women appears to be the most cost-effective strategy.[51][52]
The charitable organization Group B Strep Support have published a list of hospitals in the UK that offer the detection of GBS using the enrichment broth culture method (enrichment culture medium, ECM).[70] This test is also available privately from around £35 per test for a home-testing pack, and it is offered by private clinics.[70] The test is also available privately, for a UK-wide postal service.[71] [72]
Point-of-care testing
No current culture-based test is both accurate enough and fast enough to be recommended for detecting GBS once labour starts. Plating of swab samples requires time for the bacteria to grow, meaning that this is unsuitable to be used as an intrapartum point-of-care test.
Alternative methods to detect GBS in clinical samples (as vaginorectal swabs) rapidly have been developed, such are the methods based on nucleic acid amplification tests, such as polymerase chain reaction (PCR) tests, and DNA hybridization probes. These tests can also be used to detect GBS directly from broth media, after the enrichment step, avoiding the subculture of the incubated enrichment broth to an appropriate agar plate.[16][20][73]
Testing women for GBS colonization using vaginal or rectal swabs at 35–37 weeks of gestation and culturing them in an enriched media is not as rapid as a PCR test that would check whether the pregnant woman is carrying GBS at delivery. PCR tests would allow starting IAP on admission to the labour ward in those women for whom it is not known if they are GBS carriers.[20] PCR testing for GBS carriage could, in the future, be sufficiently accurate to guide IAP. However, the PCR technology to detect GBS must be improved and simplified to make the method cost-effective and fully useful as a point-of-care test. These tests still cannot replace antenatal culture for the accurate detection of GBS.[20][22][74] Nevertheless, point-of-care testing may be used for women who present in labor with an unknown GBS status and without risk factors for ascertaining the use of IAP.[22]
Missed opportunities of prevention
The important factors for successful prevention of GBS-EOD using IAP and the universal screening approach are:
- Reach most pregnant women for antenatal screens
- Proper sample collection
- Using an appropriate procedure for detecting GBS
- Administering a correct IAP to GBS carriers
Most cases of GBS-EOD occur in term infants born to mothers who screened negative for GBS colonization and in preterm infants born to mothers who were not screened, though some false-negative results observed in the GBS screening tests can be due to the test limitations and to the acquisition of GBS between the time of screening and delivery. These data show that improvements in specimen collection and processing methods for detecting GBS are still necessary in some settings. False-negative screening test, along with failure to receive IAP in women delivering preterm with unknown GBS colonization status, and the administration of inappropriate IAP agents to penicillin-allergic women account for most missed opportunities for prevention of cases of GBS-EOD.
GBS-EOD infections presented in infants whose mothers had been screened as GBS culture-negative are particularly worrying, and may be caused by incorrect sample collection, delay in processing the samples, incorrect laboratory techniques, recent antibiotic use, or GBS colonization after the screening was carried out.[48][75][76][77][78]
Epidemiology
In 2000–2001, the reported overall incidence of GBS infection in newborn babies in the UK was 0.72 per 1,000 live births, 0.47 per 1,000 for GBS-EOD and 0.25 per 1,000 for GBS-LOD. Very marked variations were observed, the incidence in Scotland was 0.42 per 1,000, whilst in Northern Ireland, it was 0.9 per 1,000 live births. [79][80]
Nevertheless, it may be a serious underestimation of the real incidence of GBS infections in newborns. A plausible explanation of this is that a considerable number of infants with probable GBS-EOD had negative cultures as a result of a previous maternal antibiotic treatment that inhibits the growth of GBS in blood and cerebrospinal fluid cultures, but does not mask clinical symptoms.[81][82]
Data collected prospectively for neonates that required a septic screen in the first 72 hrs of life in the UK, indicated a combined rate of definite and probable GBS-EOD infection of 3.6 per 1,000 live births. [83] Another study on the epidemiology of invasive GBS infections in England and Wales, reported a rise in the incidence of GBS-EOD between 2000 and 2010 from 0.28 to 0.41 per 1,000 live births. Rates of GBS-LOD also increased between 1991 and 2010 from 0.11 to 0.29 per 1,000 live births in England and Wales.[84]
In the past, the incidence of GBS-EOD ranged from 0.7 to 3.7 per thousand live births in the US,[5] and from 0.2 to 3.25 per thousand in Europe.[24] In 2008, after widespread use of antenatal screening and intrapartum antibiotic prophylaxis, the Centers for Disease Control and Prevention in the United States reported an incidence of 0.28 cases of GBS-EOD per thousand live births in the US.[85] From 2006 to 2015 the incidence of GBS EOD decresed to 0.37 to 0.23 per thousand live births in the US.[86] In contrast, the incidence of GBS-LOD has remained unchanged at 0.26-0.31 per 1,000 live births in the US.[86][87]

In Spain, the incidence of GBS vertical sepsis declined by 73.6%, from 1.25/1,000 live births in 1996 to 0.33/1,000 in 2008.[88] In the Barcelona area between 2004 and 2010, the incidence of GBS-EOD was 0.29 per thousand living newborns, with no significant differences along the years. The mortality rate was 8.16%.[48][89]
In France since 2001, a rapid decrease in the incidence of the neonatal GBS infections has also been reported after widespread use of IAP, from 0.7 to 0.2 per 1,000 births between 1997 and 2006.[90]
Since 2012 the incidence of neonatal GBS infection has been estimated as 0.53 per 1,000 births in the European region, 0.67 in America, and 0.15 in Australasia. Countries reporting no use of IAP had a 2.2-fold higher incidence of GBS-EOD compared with those reporting any use of IAP.[34][80]
It has been estimated that GBS infections cause at least 409.000 maternal/fetal/infant cases and 147.000 stillbirths and infant deaths worldwide annually.[91]
The following are estimates of the chances that a baby will be infected with a GBS neonatal infection if no preventive measures are taken and no other risk factors are present: [92]
- One in 1,000 where the woman is not a known GBS carrier
- One in 400 where the woman carries GBS during the pregnancy
- One in 300 where the woman carries GBS at delivery
- One in 100 where the woman had a previous baby infected with GBS
If a woman who carries GBS is given IAP during labour, the baby's risk is reduced significantly:
- One in 8,000 where the mother carries GBS during pregnancy;
- One in 6,000 where the mother carries GBS at delivery; and
- One in 2,200 where the mother has previously had a baby infected with GBS
Guidelines
United Kingdom
Royal College of Obstetricians and Gynaecologists (RCOG)
The Royal College of Obstetricians and Gynaecologists (RCOG) issued their Green Top Guideline No 36 "Prevention of early onset neonatal Group B streptococcal disease" in 2003. This guideline clearly stated: "Routine bacteriological screening of all pregnant women for antenatal GBS carriage is not recommended, and vaginal swabs should not be taken during pregnancy unless there is a clinical indication to do so." But, "Intrapartum antibiotic prophylaxis should be offered if GBS is detected on a vaginal swab in the current pregnancy."
Nevertheless, this guideline uses minimum incidence figures from a study undertaken in 2000-2001, [93] so it could not only have underestimated the true incidence of GBS infection, but it could also have underestimated the risks to babies from GBS infection. GBS infection in babies has increased in England, Wales, and Northern Ireland since 2003 (when the guideline was introduced). Voluntarily reported cases from the Communicable Disease Report/Health Protection Agency show 0.48 cases per 1,000 live births in 2003, and this figure increased to 0.64 per 1,000 in 2009.[94]
In 2007, the RCOG published the findings of their audit to evaluate practice in the UK obstetric units against their recommendations.[95] The audit started out by comparing international guidelines for prevention of GBS-EOD: highlighting the fact that, in contrast to the UK and New Zealand guidelines, most of the other countries recommended identifying women for IAP by offering effective tests to all pregnant women. The audit reviewed hospitals' protocols against GBS infection in newborns. Of the 161 UK units, which submitted their protocol, four units did not even have a protocol for GBS, of those that did, 35% did not mention the 2003 RCOG guideline, and only a minority of units had protocols entirely consistent with the guideline.
Further UK research published in 2010 looked at the opportunities for prevention of GBS-EOD following the introduction of the RCOG guideline. They found that, in the 48 cases of GBS during 2004 to 2007 (0.52/1,000 live births), only 19% of the mothers in whom risk factors were present were given adequate IAP. The researchers stated: "if all women with risk factors received prophylaxis, 23 cases (48%) may have been prevented."[53]
The 2003 RCOG guideline was reviewed in July 2012, but no substantial changes were made. The most notable change being the clarification of procedure when a woman carrying GBS has PROM and the clarification that oral antibiotics are not recommended in labour against GBS infection in the baby.
The review also dealt with a common misconception regarding vaginal cleansing stating that no evidence shows that this procedure can reduce GBS infection in the baby. New evidence and guidance in this field were reviewed by the RCOG in 2014, and it was decided that revision of the guideline would be deferred to a later date and in the meantime the version available on the website will remain valid until replaced.
The second and final audit report into GBS (Audit of current practice in preventing GBS EOD in the UK) has been published. As a result of the audit, the RCOG have recommended that the national guidelines for preventing GBS infection in newborns should be updated.[96]
In the UK, the RCOG still does not recommend bacteriological screening of pregnant women for antenatal GBS carriage in its revised new guidelines.[59] Nevertheless it is stated that if GBS carriage is detected incidentally or by intentional testing, women should be offered IAP. And that all pregnant women should be provided with an appropriate information leaflet about GBS and pregnancy (published in December 2017).[97] Instead, women are treated according to their risk in labour. IAP is given to women where GBS has been found from their urine or vaginal/rectal swabs taken during the pregnancy, and to women who have previously had a baby with GBS disease. Immediate induction of labour and IAP should be offered to all women with prelabour rupture of membranes at 37 weeks of gestation or more, to women whose membranes are ruptured more than 18 hours and to those who have fever in labour.
Women who are pyrexial in labour should be offered broad-spectrum antibiotics including an antibiotic appropriate for preventing EOD-GBS.[59]
In the UK, it has also been suggested that: "For women known to carry GBS where it is not expected that the intravenous antibiotics can be given for at least 4 hours before delivery, an intramuscular injection of 4.8 MU (2.9 g) of Penicillin G at about 35 weeks of pregnancy may be useful in addition to intravenous antibiotics given from the onset of labour or membranes rupturing until delivery to try to eradicate GBS carriage until after delivery". [98] However, this recommendation IS NOT supported by any of the present guidelines.[20][22][59]
NICE guidelines
The UK's National Institute for Health and Care Excellence (NICE) does not recommend routine testing for GBS, stating: "Pregnant women should not be offered routine antenatal screening for group B streptococcus because evidence of its clinical and cost effectiveness remains uncertain."[99]
Nevertheless, the NICE guideline "Neonatal infection: antibiotics for prevention and treatment" states: "Intrapartum Antibiotic Prophylaxis should be offered if group B streptococcal colonisation, bacteriuria or infection are detected in the current pregnancy".[100]
National Screening Committee
The UK National Screening Committee's current policy position on GBS is: "screening should not be offered to all pregnant women. This policy was reviewed in 2012, and despite receiving 212 responses, of which 93% advocated screening, the NSC has decided to not recommend antenatal screening.[101]
This decision was strongly criticized by the charity Group B Strep Support as ignoring both the wishes of the public and the rising incidence rates of GBS infection in the UK.[102]
In May 2006, the UK National Screening Committee launched their GBS online learning package. This learning package was developed to raise awareness of GBS amongst health care professionals. Developed by the Women's Health Specialist Library (part of the National Library for Health), the learning package was based upon the current UK guidelines published by the RCOG, and it is divided into three sections – antenatal, delivery, and postnatal. Within each section, the option exists to access an introduction to GBS, different clinical scenarios, a series of quiz questions to test knowledge, and a FAQs section.
United States
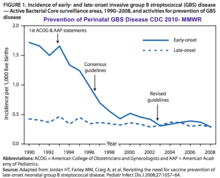
Recommendations for IAP to prevent perinatal GBS disease were issued in 1996 by the CDC. In these guidelines, the use of one of two prevention methods was recommended: either a risk-based approach or a culture-based screening approach.[45] The CDC issued updated guidelines in 2002; these guidelines recommended the universal culture-based screening of all pregnant women at 35–37 weeks' gestation to optimize the identification of women who must receive IAP. CDC also recommended that women with unknown GBS colonization status at the time of delivery be managed according to the presence of intrapartum risk factors. Because of this strategy, the US has seen a major reduction in the incidence of GBS-EOD. [103]
The CDC issued updated guidelines again in 2010, however, the foundations of prevention in the CDC's 2010 guidelines remained unchanged.[20] The following were the main additions in the 2010 guidelines:
- Expanded options for laboratory detection of GBS include the use of pigmented media and PCR assays.
- A revised colony count threshold was set for laboratories to report GBS in the urine of pregnant women.
- Revised algorithms for GBS screening and use of IAP for women with threatened preterm delivery include one algorithm for preterm labor and one for preterm premature rupture of membranes.
- Recommendations for IAP agents are presented in an algorithm format in an effort to promote the use of the most appropriate antibiotic for penicillin-allergic women.
- A minor change has been made to penicillin dosing to facilitate implementation in facilities with different packaged penicillin products.
- The neonatal management algorithm's scope was expanded to apply to all newborns.
- Management recommendations depend upon clinical appearance of the neonate and other risk factors such as maternal chorioamnionitis, adequacy of IAP if indicated for the mother, gestational age, and duration of membrane rupture.
- Changes were made to the algorithm to reduce unnecessary evaluations in well-appearing newborns at relatively low risk for GBS-EOD.
In 2018, the task of revising and updating the GBS prophylaxis guidelines were transferred from the CDC to ACOG (American College of Obstetricians and Gynecologists) (ACOG) and to the American Academy of Pediatrics.
The ACOG committee issued an updated document on Prevention of Group B Streptococcal Early-Onset Disease in Newborns in 2019.[22] ACOG’s guidance replaced the 2010 guidelines published by CDC.[104]
This document does not introduce important changes from the CDC guidelines. The key measures necessary for preventing neonatal GBS early onset disease continue to be universal prenatal screening by culture of GBS from swabs collected from the lower vagina and rectum, correct collection and microbiological processing of the samples, and proper implementation of intrapartum antibiotic prophylaxis. It is also important to note that the ACOG recommended performing universal GBS screening between 36 and 37 weeks of gestation. This new recommendation provides a 5-week window[60] for valid culture results that includes births that occur up to a gestational age of at least 41 weeks.
In 2019, American Academy of Pediatrics (AAP) published a new clinical report—Management of Infants at Risk for GBS neonatal disease.[105] AAP’s Clinical Report replaced the 2010 guidelines published by CDC.
Other guidelines
National guidelines in most developed countries advocate the use of universal screening of pregnant women late in pregnancy to detect GBS carriage and use of IAP in all colonized mothers. e.g. Canada,[106] Spain,[107] Switzerland,[108] Germany,[109] Poland,[110] Czech Republic,[111] France,[112] Norway, and Belgium.[113]
In contrast, risk factor-based guidelines were issued in the Netherlands,[114] New Zealand, Argentina,[115] and Queensland. [116] Nevertheless, the Royal Australian and New Zealand College of Obstetricians and Gynaecologists does not recommend clearly one of both prevention strategies -either the risk-based or the culture-based approach to identify pregnant women for IAP, and allow practitioners to choose according jurisdictional guidelines.[117]
Adults
GBS is also an important infectious agent able to cause invasive infections in adults. Serious life-threatening invasive GBS infections are increasingly recognized in the elderly and in individuals compromised by underlying diseases such as diabetes, cirrhosis and cancer. GBS infections in adults include urinary tract infection, skin and soft-tissue infection (skin and skin structure infection) bacteremia without focus, osteomyelitis, meningitis and endocarditis.[14] GBS infection in adults can be serious, and mortality is higher among adults than among neonates.[118] In general, penicillin is the antibiotic of choice for treatment of GBS infections. Erythromycin or clindamycin should not be used for treatment in penicillin-allergic patients unless susceptibility of the infecting GBS isolate to these agents is documented. Gentamicin plus penicillin (for antibiotic synergy) in patients with life-threatening GBS infections may be used.[119][120][121]
Toxic shock syndrome (TSS) is an acute multisystem life-threatening disease resulting in multiple organ failure. The severity of this disease frequently warrants immediate medical treatment. TSS is caused primarily by some strains of Staphylococcus aureus and Streptococcus pyogenes that produce exotoxins. Nevertheless, invasive GBS infection can be complicated, though quite infrequently, by streptococcal toxic shock-like syndrome (STLS)[122]
Society and culture
July has been recognised as Group B Strep Awareness Month,[123] a time when information about group B Strep aimed at families and health professionals is shared, predominantly in the UK and the US. In the UK, this is led by Group B Strep Support[124]
Vaccine
Though the introduction of national guidelines to screen pregnant women for GBS carriage and the use of IAP has significantly reduced the burden of GBS-EOD disease, it has had no effect on preventing either GBS-LOD in infants or GBS infections in adults.[125] Because of this if an effective vaccine against GBS were available, it would be an effective means of controlling not only GBS disease in infants, but also infections in adults.
There are a number of problems with giving antibiotics to women in labor. Such antibiotic exposure risks included severe allergic reactions and difficulties screening pregnant women for GBS. If pregnant women could be given a vaccine against GBS, this could potentially prevent most cases of GBS without the need for antibiotics or screening. Vaccination is considered an ideal solution to prevent not only early- and late-onset disease but also GBS infections in adults at risk.[126]
Development of GBS vaccines for maternal immunization has been identified as a priority by the World Health Organization on the basis of high unmet need.[127] It has been estimated that such a vaccine could potentially prevent 231,000 infant and maternal GBS cases.[128]
As early as 1976,[32] low levels of maternal antibodies against the GBS capsular polysaccharide were shown to be correlated with susceptibility to GBS-EOD and GBS-LOD. Maternal-specific antibodies, transferred from the mother to the newborn, were able to confer protection to babies against GBS infection.[129] The capsular polysaccharide of GBS, which is an important virulence factor, is also an excellent candidate for the development of an effective vaccine.[130][129][131][132]
GBS protein-based vaccines are also in development.[133][134][135]
At present, the licensing of GBS vaccines is difficult because of the challenge in conducting clinical trials in humans due to the low incidence of GBS neonatal diseases.[24][131][136] Nevertheless, though research and clinical trials for the development of an effective vaccine to prevent GBS infections are underway, no vaccine is available as of 2019.[133][137]
Nonhuman infections
GBS has been found in many mammals and other animals such as camels, dogs, cats, seals, dolphins, and crocodiles.[138]
Cattle
In cattle, GBS causes mastitis, an infection of the udder. It can produce an acute febrile disease or a subacute, more chronic disease. Both lead to diminishing milk production (hence its name: agalactiae meaning "no milk"). Mastitis associated with GBS can have an important effect on the quantity and quality of milk produced, and is also associated with elevated somatic cell count and total bacteria count in the milk.[139] Outbreaks in herds are common, and as this is of major significance for the dairy industry, programs to reduce the impact of GBS have been enforced in many countries[140]
Fish
GBS it is also an important pathogen in a diversity of fish species, leading to serious economic losses in many species of fish worldwide. GBS causes severe epidemics in farmed fish, causing sepsis and external and internal hemorrhages. GBS infection has been reported from wild and captive fish and has been involved in epizootics in many countries.[141][142] Vaccines to protect fish against GBS infections are under development.[143][144]
References
- "Group B strep". nhs.uk. 7 February 2018. Retrieved 8 December 2019.
- Maisey HC, Doran KS, Nizet V (2009). "Recent advances in understanding the molecular basis of group B Streptococcus virulence". Expert Reviews in Molecular Medicine. 10: e27. doi:10.1017/S1462399408000811. PMC 2676346. PMID 18803886.
- Rajagopal L. (2009). "Understanding the regulation of Group B Streptococcal virulence factors". Future Microbiology. 4 (2): 201–221. doi:10.2217/17460913.4.2.201. PMC 2691590. PMID 19257847.
- Leclercq SY, Sullivan MJ, Ipe DS, Smith JP, Cripps AW, Ulett GC (2016). "Pathogenesis of Streptococcus urinary tract infection depends on bacterial strain and β-hemolysin/cytolysin that mediates cytotoxicity, cytokine synthesis, inflammation and virulence". Scientific Reports. 6: 29000. Bibcode:2016NatSR...629000L. doi:10.1038/srep29000. PMC 4935997. PMID 27383371.
- Edwards MS, Nizet V (2011). Group B streptococcal infections. Infectious Diseases of the Fetus and Newborn Infant (7th. ed.). Elsevier. pp. 419–469. ISBN 978-0-443-06839-3.
- Whidbey C, Harrell MI, Burnside K, Ngo L, Becraft AK, Iyer LM, Aravind L, Hitti J, Waldorf KM, Rajagopal L (2013). "A hemolytic pigment of Group B Streptococcus allows bacterial penetration of human placenta". The Journal of Experimental Medicine. 210 (6): 1265–1281. doi:10.1084/jem.20122753. PMC 3674703. PMID 23712433.
- Rosa-Fraile M, Dramsi S, Spellerberg B (2014). "Group B streptococcal haemolysin and pigment, a tale of twins" (PDF). FEMS Microbiology Reviews. 38 (5): 932–946. doi:10.1111/1574-6976.12071. PMC 4315905. PMID 24617549.
- Whidbey C, Vornhagen J, Gendrin C, Boldenow E, Samson JM, Doering K, Ngo L, Ezekwe EA Jr, Gundlach JH, Elovitz MA, Liggitt D, Duncan JA, Adams Waldorf KM, Rajagopal L (2015). "A streptococcal lipid toxin induces membrane permeabilization and pyroptosis leading to fetal injury". EMBO Molecular Medicine. 7 (4): 488–505. doi:10.15252/emmm.201404883. PMC 4403049. PMID 25750210.
- Christopher-Mychael Whidbey (2015). Characterization of the Group B Streptococcus Hemolysin and its Role in Intrauterine Infection (PDF). University of Washington.
- Keefe GP. (1997). "Streptococcus agalactiae mastitis: A review". The Canadian Veterinary Journal. 38 (7): 199–204. PMC 1576741. PMID 9220132.
- Fry RM. (1938). "Fatal infections by haemolytic streptococcus group B.". The Lancet. 231 (5969): 199–201. doi:10.1016/S0140-6736(00)93202-1.
- Eickhoff TC; Klein JO; Kathleen Daly A; David Ingall; Finland M. (1964). "Neonatal Sepsis and Other Infections Due to Group B Beta-Hemolytic Streptococci". New England Journal of Medicine. 271 (24): 1221–1228. doi:10.1056/NEJM196412102712401. PMID 14234266.
- "Group B Strep Infection". MedicineNet.com. Retrieved 10 January 2016.
- Edwards MS, Baker CJ (2010). Streptococcus agalactiae (group B streptococcus). Mandell GL, Bennett JE, Dolin R (eds) Principles and practice of infectious diseases. Vol 2 (7th. ed.). Elsevier. pp. Chapter 202. ISBN 978-0-443-06839-3.
- Tille P. (2014). Bailey & Scott's Diagnostic Microbiology (13th. ed.). Elsevier. ISBN 978-0-323-08330-0.
- Rosa-Fraile M.,Spellerberg B. (2017). "Reliable Detection of Group B Streptococcus in the Clinical Laboratory" (PDF). Journal of Clinical Microbiology. 55 (9): 2590–2598. doi:10.1128/JCM.00582-17. PMC 5648696. PMID 28659318. Retrieved 23 November 2019.
- Rosa-Fraile M, Rodriguez-Granger J, Cueto-Lopez M, Sampedro A, Biel Gaye E, Haro M, Andreu A (1999). "Use of Granada medium to detect group B streptococcal colonization in pregnant women". Journal of Clinical Microbiology. 37 (8): 2674–2677. doi:10.1128/JCM.37.8.2674-2677.1999. PMC 85311. PMID 10405420.
- Binghuai L, Yanli S, Shuchen Z, Fengxia Z, Dong L, Yanchao C (2014). "Use of MALDI-TOF mass spectrometry for rapid identification of group B Streptococcus on chromID Strepto B agar". International Journal of Infectious Diseases. 27: 44–48. doi:10.1016/j.ijid.2014.06.023. PMID 25220051.
- To KN, Cornwell E, Daniel R, Goonesekera S, Jauneikaite E, Chalker V, Le Doare K. (2019). "Evaluation of matrix-assisted laser desorption ionisation time-of-flight mass spectrometry (MALDI-TOF MS) for the Identification of Group B Streptococcus". BMC Research Notes. 12 (1): 85. doi:10.1186/s13104-019-4119-1. PMC 6376729. PMID 30764872.CS1 maint: multiple names: authors list (link)
- Verani JR, McGee L, Schrag SJ (2010). "Prevention of perinatal group B streptococcal disease: revised guidelines from CDC" (PDF). MMWR Recomm. Rep. 59 ((RR-10)): 1–32.CS1 maint: multiple names: authors list (link)
- El Aila NA, Tency I, Claeys G, Saerens B, Cools P, Verstraelen H, Temmerman M, Verhelst R, Vaneechoutte M (2010). "Comparison of different sampling techniques and of different culture methods for detection of group B streptococcus carriage in pregnant women". BMC Infectious Diseases. 10: 285. doi:10.1186/1471-2334-10-285. PMC 2956727. PMID 20920213.CS1 maint: multiple names: authors list (link)
- American College of Obstetricians and Gynecologists (ACOG). (2019). "Prevention of Group B Streptococcal Early-Onset Disease in Newborns ACOG Committee Opinion, Number 782". Obstetrics and Gynecology. 134 (1): e19-40. doi:10.1097/AOG.0000000000003334. PMID 31241599. S2CID 195659363.
- Barcaite E, Bartusevicius A, Tameliene R, Kliucinskas M, Maleckiene L, Nadisauskiene R (2008). "Prevalence of maternal group B streptococcal colonisation in European countries". Acta Obstetricia et Gynecologica Scandinavica. 87 (3): 260–271. doi:10.1080/00016340801908759. PMID 18307064.
- Rodriguez-Granger J, Alvargonzalez JC, Berardi A, Berner R, Kunze M, Hufnagel M, Melin P, Decheva A, Orefici G, Poyart C, Telford J, Efstratiou A, Killian M, Krizova P, Baldassarri L, Spellerberg B, Puertas A, Rosa-Fraile M (2012). "Prevention of group B streptococcal neonatal disease revisited. The DEVANI European project". European Journal of Clinical Microbiology & Infectious Diseases. 31 (9): 2097–2114. doi:10.1007/s10096-012-1559-0. PMID 22314410. S2CID 15588906.
- Muller AE, Oostvogel PM, Steegers EA, Dörr PJ. (2016). "Morbidity related to maternal group B streptococcal infections". Acta Obstetricia et Gynecologica Scandinavica. 85 (9): 1027–37. doi:10.1080/00016340600780508. PMID 16929406.CS1 maint: multiple names: authors list (link)
- Cunningham, F, Leveno KJ, Bloom SL, Spong CY, Dashe JS, Hoffman BL, Casey BM, Sheffield JS (2013). "Abortion". Williams Obstetrics. McGraw-Hill. p. 5.
- Libster R, Edwards KM, Levent F, Edwards MS, Rench MA, Castagnini LA, Cooper T, Sparks RC, Baker CJ, Shah PE (2012). "Long-term outcomes of group B streptococcal meningitis" (PDF). Pediatrics. 130 (1): e8–15. doi:10.1542/peds.2011-3453. PMID 22689869. S2CID 1013682.
- Polin RA. (2012). "Management of Neonates With Suspected or Proven Early-Onset Bacterial Sepsis" (PDF). Pediatrics. 129 (5): 1006–1015. doi:10.1542/peds.2012-0541. PMID 22547779. S2CID 230591.
- Martinez E, Mintegi S, Vilar B, Martinez MJ, Lopez A, Catediano E, Gomez B (2015). "Prevalence and predictors of bacterial meningitis in young infants with fever without a source". The Pediatric Infectious Disease Journal. 34 (5): 494–498. doi:10.1097/inf.0000000000000629. PMID 25461476.
- Boyer KM, Gotoff SP (1985). "Strategies for Chemoprophylaxis of GBS Early-Onset Infections1". Strategies for chemoprophylaxis of GBS early-onset infections. Antibiotics and Chemotherapy. 35. pp. 267–280. doi:10.1159/000410380. ISBN 978-3-8055-3953-1. PMID 3931544.
- Dauby N, Chamekh M, Melin P, Slogrove A, Goetghebuer T (2016). "Increased Risk of Group B Streptococcus Invasive Infection in HIV-Exposed but Uninfected Infants: A Review of the Evidence and Possible Mechanisms". Frontiers in Immunology. 16: 505. doi:10.3389/fimmu.2016.00505. PMC 5110531. PMID 27899925.
- Baker CJ, Kasper DL (1976). "Correlation of maternal antibody deficiency with susceptibility to neonatal infection with group B Streptococcus". The New England Journal of Medicine. 294 (14): 753–756. doi:10.1056/nejm197604012941404. PMID 768760.
- Baker CJ, Edwards MS, Kasper DL (1981). "Role of antibody to native type III polysaccharide of group B Streptococcus in infant infection". Pediatrics. 68 (4): 544–549. PMID 7033911.
- Edmond KM, Kortsalioudaki C, Scott S, Schrag SJ, Zaidi AK, Cousens S, Heath PT (2012). "Group B streptococcal disease in infants aged younger than 3 months: systematic review and meta-analysis" (PDF). The Lancet. 379 (9815): 547–556. doi:10.1016/s0140-6736(11)61651-6. PMID 22226047.
- Lin FY, Weisman LE, Troendle J, Adams K (2003). "Prematurity Is the Major Risk Factor for Late-Onset Group B Streptococcus Disease" (PDF). The Journal of Infectious Diseases. 188 (2): 267–271. doi:10.1086/376457. PMID 12854082.
- Lin, F; Brenner, RA; Johnson, YR; Azimi, PH; Philips Jb, 3rd; Regan, JA; Clark, P; Weisman, LE; et al. (2001). "The effectiveness of risk-based intrapartum chemoprophylaxis for the prevention of early-onset neonatal group B streptococcal disease". American Journal of Obstetrics and Gynecology. 184 (6): 1204–10. doi:10.1067/mob.2001.113875. PMID 11349189.
- De Cueto, M; Sanchez, MJ; Sampedro, A; Miranda, JA; Herruzo, AJ; Rosa-Fraile, M (1998). "Timing of Intrapartum Ampicillin and Prevention of Vertical Transmission of Group B Streptococcus". Obstetrics & Gynecology. 91 (1): 112–4. doi:10.1016/S0029-7844(97)00587-5. PMID 9464732. S2CID 22858678.
- Berardi A, Rossi C, Biasini A, Minniti S, Venturelli C, Ferrari F, Facchinetti F (2011). "Efficacy of intrapartum chemoprophylaxis less than 4 hours duration". The Journal of Maternal-Fetal & Neonatal Medicine. 24 (4): 619–625. doi:10.3109/14767058.2010.511347. PMID 20828241. S2CID 6697604.
- Bhattacharya S. (2010). "The facts about Penicillin Allergy: A Review". Journal of Advanced Pharmaceutical Technology & Research. 1 (1): 11–17. PMC 3255391. PMID 22247826.
- Baltimore RS, Huie SM, Meek JI, Schuchat A, O'Brien KL (2001). "Early-onset neonatal sepsis in the era of group B streptococcal prevention". Pediatrics. 108 (5): 1094–1098. doi:10.1542/peds.108.5.1094. PMID 11694686.
- Sutkin G, Krohn MA, Heine RP, Sweet RL (2005). "Antibiotic prophylaxis and non-group B streptococcal neonatal sepsis". Obstetrics & Gynecology. 105 (3): 581–586. doi:10.1097/01.aog.0000153492.30757.2f. PMID 15738028.
- Schrag SJ, Hadler JL, Arnold KE, Martell-Cleary P, Reingold A, Schuchat A (2006). "Risk factors for invasive, early-onset Escherichia coli infections in the era of widespread intrapartum antibiotic use". Pediatrics. 118 (2): 560–566. doi:10.1542/peds.2005-3083. PMID 16882809. S2CID 34908773.
- Cutland, Clare L; Madhi, Shabir A; Zell, Elizabeth R; Kuwanda, Locadiah; Laque, Martin; Groome, Michelle; Gorwitz, Rachel; Thigpen, Michael C; et al. (2009). "Chlorhexidine maternal-vaginal and neonate body wipes in sepsis and vertical transmission of pathogenic bacteria in South Africa: A randomised, controlled trial". The Lancet. 374 (9705): 1909–16. doi:10.1016/S0140-6736(09)61339-8. PMID 19846212. S2CID 23418670.
- Ohlsson, A; Shah, VS; Stade, BC (14 December 2014). "Vaginal chlorhexidine during labour to prevent early-onset neonatal group B streptococcal infection". Cochrane Database of Systematic Reviews. 12 (12): CD003520. doi:10.1002/14651858.CD003520.pub3. PMID 25504106.
- CDC (1996). "Prevention of Perinatal Group B Streptococcal Disease: A Public Health Perspective". MMWR. 45-RR7: 1–24.
- Clifford V, Garland SM, Grimwood K (2011). "Prevention of neonatal group B streptococcus disease in the 21st century". Journal of Paediatrics and Child Health. 48 (9): 808–815. doi:10.1111/j.1440-1754.2011.02203.x. PMID 22151082.
- Schrag SJ, Zell ER, Lynfield R, Roome A, Arnold KE, Craig AS, Harrison LH, Reingold A, Stefonek K, Smith G, Gamble M, Schuchat A; Active Bacterial Core Surveillance Team. (2002). "A population-based comparison of strategies to prevent early-onset group B streptococcal disease in neonates". New England Journal of Medicine. 347 (4): 233–239. doi:10.1056/nejmoa020205. PMID 12140298.CS1 maint: multiple names: authors list (link)
- Giménez M, Sanfeliu I, Sierra M, Dopico E, Juncosa T, Andreu A, Lite J, Guardià C, Sánchez F, Bosch J., Article in Spanish. (2015). "Evolución de la sepsis neonatal precoz por Streptococcus agalactiae en el área de Barcelona (2004-2010). Análisis de los fallos del cumplimiento del protocolo de prevención. Group B streptococcal early-onset neonatal sepsis in the area of Barcelona (2004-2010). Analysis of missed opportunities for prevention" (PDF). Enfermedades Infecciosas y Microbiologia Clinica. 33 (7): 446–450. doi:10.1016/j.eimc.2014.10.015. PMID 25541009. Archived from the original (PDF) on 23 February 2016. Retrieved 15 January 2016.CS1 maint: multiple names: authors list (link)
- Phares CR, Lynfield R, Farley MM, Mohle-Boetani J, Harrison LH, Petit S, Craig AS, Schaffner W, Zansky SM, Gershman K, Stefonek KR, Albanese BA, Zell ER, Schuchat A, Schrag SJ; Active Bacterial Core surveillance/Emerging Infections Program Network. (2008). "Epidemiology of Invasive Group B Streptococcal Disease in the United States, 1999-2005". JAMA. 299 (17): 2056–2065. doi:10.1001/jama.299.17.2056. PMID 18460666.CS1 maint: multiple names: authors list (link)
- Colbourn, T; Asseburg, C; Bojke, L; Philips, Z; Claxton, K; Ades, AE; Gilbert, RE (2007). "Prenatal screening and treatment strategies to prevent group B streptococcal and other bacterial infections in early infancy: Cost-effectiveness and expected value of information analyses". Health Technology Assessment. 11 (29): 1–226, iii. doi:10.3310/hta11290. PMID 17651659.
- Colbourn, T. E; Asseburg, C.; Bojke, L.; Philips, Z.; Welton, N. J; Claxton, K.; Ades, A E; Gilbert, R. E (2007). "Preventive strategies for group B streptococcal and other bacterial infections in early infancy: Cost effectiveness and value of information analyses". BMJ. 335 (7621): 655. doi:10.1136/bmj.39325.681806.AD. PMC 1995477. PMID 17848402.
- Kaambwa B, Bryan S, Gray J, Milner P, Daniels J, Khan KS, Roberts TE (2010). "Cost-effectiveness of rapid tests and other existing strategies for screening and management of early-onset group B streptococcus during labour". BJOG: An International Journal of Obstetrics & Gynaecology. 117 (13): 1616–1627. doi:10.1111/j.1471-0528.2010.02752.x. PMID 21078057.
- Vergnano S, Embleton N, Collinson A, Menson E, Bedford Russell A, Heath P (2010). "Missed opportunities for preventing group B streptococcus infection". Archives of Disease in Childhood - Fetal and Neonatal Edition. 95 (1): F72–73. doi:10.1136/adc.2009.160333. PMID 19439431. S2CID 38297857.
- Steer, P.J.; Plumb, J. (2011). "Myth: Group B streptococcal infection in pregnancy: Comprehended and conquered". Seminars in Fetal and Neonatal Medicine. 16 (5): 254–8. doi:10.1016/j.siny.2011.03.005. PMID 21493170.
- Siegel JD, Cushion NB (1996). "Prevention of early-onset group B streptococcal disease: another look at single-dose penicillin at birth". Obstetrics & Gynecology. 87 (5 Pt 1): 692–698. doi:10.1016/0029-7844(96)00004-x. PMID 8677068.
- Velaphi S, Siegel JD, Wendel GD Jr, Cushion N, Eid WM, Sanchez PJ (2003). "Early-onset group B streptococcal infection after a combined maternal and neonatal group B streptococcal chemoprophylaxis strategy". Pediatrics. 111 (3): 541–547. doi:10.1542/peds.111.3.541. PMID 12612234.
- Woodgate PG, Flenady V, Steer PA (2004). "Intramuscular penicillin for the prevention of early onset group B streptococcal infection in newborn". The Cochrane Database of Systematic Reviews (3): CD003667. doi:10.1002/14651858.CD003667.pub2. PMID 15266494.
- GROUP B STREP SUPPORT. "FAQs35. Carrying GBS and home birth?". Retrieved 25 November 2019.
- Hughes RG, Brocklehurst P, Steer PJ, Heath P, Stenson BM on behalf of the Royal College of Obstetricians and Gynaecologists. (2017). "Prevention of Early-onset Neonatal Group B Streptococcal Disease Green-top Guideline No. 36. September 2017". BJOG: An International Journal of Obstetrics and Gynaecology. 124 (12): e280–e305. doi:10.1111/1471-0528.14821. PMID 28901693.CS1 maint: multiple names: authors list (link)
- Yancey MK, Schuchat A, Brown LK, Ventura VL, Markenson GR. (1996). "The accuracy of late antenatal screening cultures in predicting genital group B streptococcal colonization at delivery". Obstetrics & Gynecology. 88 (5): 811–815. doi:10.1016/0029-7844(96)00320-1. PMID 8885919.CS1 maint: multiple names: authors list (link)
- Valkenburg-van den Berg AW, Houtman-Roelofsen RL, Oostvogel PM, Dekker FW, Dorr PJ, Sprij AJ (2010). "Timing of group B streptococcus screening in pregnancy: a systematic review". Gynecologic and Obstetric Investigation. 69 (3): 174–183. doi:10.1159/000265942. PMID 20016190. S2CID 26709882.
- Price D, Shaw E, Howard M, Zazulak J, Waters H, Kaczorowski J (2006). "Self-sampling for group B Streptococcus in women 35 to 37 weeks pregnant is accurate and acceptable: a randomized cross-over trial". Journal of Obstetrics and Gynaecology Canada. 28 (12): 1083–8. doi:10.1016/s1701-2163(16)32337-4. PMID 17169231.
- Hicks P, Diaz-Perez MJ (2009). "Patient self-collection of group B streptococcal specimens during pregnancy". The Journal of the American Board of Family Medicine. 22 (2): 136–140. doi:10.3122/jabfm.2009.02.080011. PMID 19264936.
- Arya A; Cryan B; O’Sullivan K; Greene RA; Higgins JR. (2008). "Self-collected versus health professional-collected genital swabs to identify the prevalence of group B streptococcus: A comparison of patient preference and efficacy". European Journal of Obstetrics & Gynecology and Reproductive Biology. 139 (1): 32–45. doi:10.1016/j.ejogrb.2007.12.005. PMID 18255214.
- Rosa-Fraile M, Camacho-Muñoz E, Rodríguez-Granger J, Liébana-Martos C (2005). "Specimen storage in transport medium and detection of group B streptococci by culture". Journal of Clinical Microbiology. 43 (2): 928–930. doi:10.1128/jcm.43.2.928-930.2005. PMC 548104. PMID 15695709.
- Carey RB. "Group B Streptococci: Chains & Changes New Guidelines for the Prevention of Early-Onset GBS" (PDF). Retrieved 11 January 2016.
- UK Gov. "SMI B 58: detection of carriage of group B streptococci. Updated 2018". Retrieved 28 November 2019.
- Gil, EG; Rodríguez, MC; Bartolomé, R; Berjano, B; Cabero, L; Andreu, A (1999). "Evaluation of the Granada agar plate for detection of vaginal and rectal group B streptococci in pregnant women". Journal of Clinical Microbiology. 37 (8): 2648–2651. doi:10.1128/JCM.37.8.2648-2651.1999. PMC 85303. PMID 10405415.
- Claeys, G.; Verschraegen, G.; Temmerman, M. (2001). "Modified Granada Agar Medium for the detection of group B streptococcus carriage in pregnant women". Clinical Microbiology and Infection. 7 (1): 22–24. doi:10.1046/j.1469-0691.2001.00156.x. PMID 11284939.
- Where can I get the ECM test?. "ECM Testing". Group B Strep Support. Retrieved 28 November 2019.
- "Group B Streptococcus Screening Test". Medisave UK Ltd. Retrieved 28 November 2019.
- "Testing for Group B Streptococcus". The Doctors Laboratory. Retrieved 28 November 2019.
- Buchan BW, Faron ML, Fuller D, Davis TE, Mayne D, Ledeboer NA (2015). "Multicenter Clinical Evaluation of the Xpert GBS LB Assay for Detection of Group B Streptococcus in Prenatal Screening Specimens". Journal of Clinical Microbiology. 53 (2): 443–448. doi:10.1128/jcm.02598-14. PMC 4298547. PMID 25411176.
- Daniels J, Gray J, Pattison H, Roberts T, Edwards E, Milner P, Spicer L, King E, Hills RK, Gray R, Buckley L, Magill L, Elliman N, Kaambwa B, Bryan S, Howard R, Thompson P, Khan KS (2009). "Rapid testing for group B streptococcus during labour: a test accuracy study with evaluation of acceptability and cost-effectiveness" (PDF). Health Technology Assessment. 13 (42): 1–154, iii–iv. doi:10.3310/hta13420. PMID 19778493. Archived from the original (PDF) on 23 February 2016.
- Melin P. (2011). "Neonatal group B streptococcal disease: from pathogenesis to preventive strategies". Clinical Microbiology and Infection. 17 (9): 1294–1303. doi:10.1111/j.1469-0691.2011.03576.x. PMID 21672083.
- Berardi A, Lugli L, Baronciani D, Rossi C, Ciccia M, Creti R, Gambini L, Mariani S, Papa I, Tridapalli E, Vagnarelli F, Ferrari F; GBS Prevention Working Group of Emilia-Romagna. (2010). "Group B Streptococcus early-onset disease in Emilia-romagna: review after introduction of a screening-based approach". The Pediatric Infectious Disease Journal. 29 (2): 115–121. doi:10.1097/inf.0b013e3181b83cd9. PMID 19915512.CS1 maint: multiple names: authors list (link)
- Schrag SJ, Verani JR (2013). "Intrapartum antibiotic prophylaxis for the prevention of perinatal group B streptococcal disease: Experience in the United States and implications for a potential group B streptococcal vaccine". Vaccine. 31S: D20–26. doi:10.1016/j.vaccine.2012.11.056. PMID 23219695.
- Pulver LS, Hopfenbeck MM, Young PC, Stoddard GJ, Korgenski K, Daly J, Byington CL (2009). "Continued early onset group B streptococcal infections in the era of intrapartum prophylaxis". Journal of Perinatology. 29 (1): 0–25. doi:10.1038/jp.2008.115. PMID 18704032.
- Heath PT, Balfour G, Weisner AM, Efstratiou A, Lamagni TL, Tighe H, O'Connell LA, Cafferkey M, Verlander NQ, Nicoll A, McCartney AC; PHLS Group B Streptococcus Working Group. (2004). "Group B streptococcal disease in UK and Irish infants younger than 90 days". The Lancet. 363 (9405): 292–294. doi:10.1016/s0140-6736(03)15389-5. PMID 14751704.CS1 maint: multiple names: authors list (link)
- Doare K, Heath PT (2013). "An overview of global GBS epidemiology". Vaccine. 31 Suppl 4: D7–12. doi:10.1016/j.vaccine.2013.01.009. PMID 23973349.
- Brigtsen A.K.; Jacobsen A.F.; Dedi L.; Melby K.K.; Fugelseth D.; Whitelaw A. (2015). "Maternal colonization with Group B Streptococcus Is associated with an increased rate of infants transferred to the neonatal intensive care unit". Neonatology. 108 (3): 157–163. doi:10.1159/000434716. PMID 26182960. S2CID 24711146.
- Carbonell-Estrany X, Figueras-Aloy J, Salcedo-Abizanda S, de la Rosa-Fraile M, Castrillo Study Group (2008). "Probable early-onset group B streptococcal neonatal sepsis: a serious clinical condition related to intrauterine infection". Archives of Disease in Childhood - Fetal and Neonatal Edition. 93 (2): F85–89. doi:10.1136/adc.2007.119958. PMID 17704105. S2CID 10300571.
- Luck, Suzanne; Torny, Michael; d'Agapeyeff, Katrina; Pitt, Alison; Heath, Paul; Breathnach, Aoadhan; Russell, Alison Bedford (2003). "Estimated early-onset group B streptococcal neonatal disease". The Lancet. 361 (9373): 1953–1954. doi:10.1016/S0140-6736(03)13553-2. PMID 12801740. S2CID 33025300.
- Lamagni TL, Keshishian C, Efstratiou A, Guy R, Henderson KL, Broughton K, Sheridan E (2013). "Emerging Trends in the Epidemiology of Invasive Group B Streptococcal Disease in England and Wales, 1991–2010". Clinical Infectious Diseases. 57 (5): 682–688. doi:10.1093/cid/cit337. PMID 23845950.
- CDC. "Group B Strep (GBS)-Clinical Overview". Retrieved 10 January 2016.
- Nanduri SA, Petit S, Smelser C, Apostol M, Alden NB, Harrison LH, Lynfield R, Vagnone PS, Burzlaff K, Spina NL, Dufort EM, Schaffner W, Thomas AR, Farley MM, Jain JH, Pondo T, McGee L, Beall BW, Schrag SJ. (2019). "Epidemiology of Invasive Early-Onset and Late-Onset Group B Streptococcal Disease in the United States, 2006 to 2015: Multistate Laboratory and Population-Based Surveillance". JAMA Pediatrics. 173 (3): 224–33. doi:10.1001/jamapediatrics.2018.4826. PMC 6439883. PMID 30640366.CS1 maint: multiple names: authors list (link)
- Baker CJ. (2013). "The spectrum of perinatal group B streptococcal disease". Vaccine. 31s: D3–6. doi:10.1016/j.vaccine.2013.02.030. PMID 23973344.
- Lopez Sastre J, Fernandez Colomer B, Coto Cotallo Gil D, Members of "Grupo de Hospitales Castrillo" (2009). "Neonatal Sepsis of Vertical Transmission. An epidemiological study from the "Grupo de Hospitales Castrillo"". Early Human Development. 85 (10): S100. doi:10.1016/j.earlhumdev.2009.08.049.CS1 maint: multiple names: authors list (link)
- Andreu A, Sanfeliu I, Viñas L, Barranco M, Bosch J, Dopico E, Guardia C, Juncosa T, Lite J, Matas L, Sánchez F, Sierr M; Grupo de Microbiólogos pare el Esduio de las Infecciones de Transmissión Vertical, Societat Catalana de Malalties Infeccioses i Microbiologia Clínica, Article in spanish. (2003). "Declive de la incidencia de la sepsis perinatal por estreptococo del grupo B (Barcelona 1994-2001). Relación con las políticas profilácticas Decreasing incidence of perinatal group B streptococcal disease (Barcelona 1994-2002). Relation with hospital prevention policies" (PDF). Enfermedades Infecciosas y Microbiologia Clinica. 21 (4): 174–179. doi:10.1157/13045447. Archived from the original (PDF) on 23 February 2016.CS1 maint: multiple names: authors list (link)
- Albouy-Llaty, Marion; Nadeau, Cédric; Descombes, Emmanuelle; Pierre, Fabrice; Migeot, Virginie (2011). "Improving perinatal Group B streptococcus screening with process indicators". Journal of Evaluation in Clinical Practice. 18 (4): 727–733. doi:10.1111/j.1365-2753.2011.01658.x. PMID 21414110.
- Seale AC, Bianchi-Jassir F, Russell NJ, Kohli-Lynch M, Tann CJ, Hall J, Madrid L, Blencowe H, Cousens S, Baker CJ, Bartlett L, Cutland C, Gravett MG, Heath PT, Ip M, Le Doare K, Madhi SA, Rubens CE, Saha SK, Schrag SJ, Sobanjo-Ter Meulen A, Vekemans J, Lawn JE. (2017). "Estimates of the Burden of Group B Streptococcal Disease Worldwide for Pregnant Women, Stillbirths, and Children". Clinical Infectious Diseases. 65 (suppl 2) (Suppl 2): S200-209. doi:10.1093/cid/cix664. PMC 5849940. PMID 29117332.CS1 maint: multiple names: authors list (link)
- Benitz WE, Gould JB, Druzin ML (1999). "Risk factors for early-onset group B streptococcal sepsis: estimation of odds ratios by critical literature review". Pediatrics. 103 (6): e77. doi:10.1542/peds.103.6.e77. PMID 10353974.
- Heath, Paul T; Balfour, Gail; Weisner, Abbie M; Efstratiou, Androulla; Lamagni, Theresa L; Tighe, Helen; O'Connell, Liam AF; Cafferkey, Mary; et al. (2004). "Group B streptococcal disease in UK and Irish infants younger than 90 days". The Lancet. 363 (9405): 292–294. doi:10.1016/S0140-6736(03)15389-5. PMID 14751704. S2CID 22371160.
- Health Protection Report Vol. 5 No. 46 – 18 November 2011 ·. "Pyogenic and non-pyogenic streptococcal bacteraemia, England, Wales and Northern Ireland: 2010". Health Protection Report. Archived from the original on 14 July 2014. Retrieved 11 January 2016.
- Cromwell D, Joffe T, van der Meulen J, Dhillon C, Hughes R, Murphy D (2007). The Prevention of Early-onset Neonatal Group B Streptococcal Disease in UK Obstetric Units (PDF). Royal College of Obstetricians and Gynaecologists and London School of Hygiene and Tropical Medicine. ISBN 978-1-904752-37-0. Retrieved 11 January 2016.
- RCOG. "Audit of current practice in preventing early-onset neonatal group B streptococcal disease in the UK" (PDF). Retrieved 2 February 2016.
- GBS Support UK & RCOG (Diciembre de 2017). "Group B Streptococcus (GBS) in pregnancy and newborn babies" (PDF). Archived from the original (PDF) on 22 December 2017. Retrieved 25 November 2019.
- Home Birth Reference Site. "Group B Strep and Home Birth". Retrieved 11 January 2016.
- Screening for infections.1.8.9 Group B streptococcus. "Antenatal care for uncomplicated pregnancies.NICE guidelines [CG62] : March 2008". NICE National Institute for Health and Care Excellence. Retrieved 27 November 2019.
- NICE guidelines [CG149] August 2012. "Neonatal infection: antibiotics for prevention and treatment. 1.3 Intrapartum antibiotics". NICE National Institute for Health and Care excellence. Retrieved 27 November 2019.
- UK National Screening Committee. "Current UK NSC from the UK National Screening Committee (UK NSC)". Retrieved 19 November 2019.
- "Leading baby charity devastated by decision not to introduce life saving screening of pregnant women.2012". campaign-archive2.com. Retrieved 30 November 2019.
- Centers for Disease Control and Prevention- CDC, MMWR (2002). "Prevention of Perinatal Group B Streptococcal Disease Revised Guidelines from CDC. 2002". Morbidity and Mortality Weekly Report. 51-RR11: 1–22. Retrieved 11 January 2016.
- CDC. "Prevention Guidelines. 2019 Guidelines Update". Retrieved 26 November 2019.
- Puopolo KM, Lynfield R, Cummings JJ; COMMITTEE ON FETUS AND NEWBORN; COMMITTEE ON INFECTIOUS DISEASES. (2019). "Management of Infants at Risk for Group B Streptococcal Disease" (PDF). Pediatrics. 144 (2): e20191881. doi:10.1542/peds.2019-1881. PMID 31285392. S2CID 195843897. Retrieved 25 November 2019.CS1 maint: multiple names: authors list (link)
- Money D, Allen VM. (2018). "No 298 - Prévention de l'infection néonatale à streptocoques du groupe B d'apparition précoce". Journal of Obstetrics and Gynaecology Canada. 40 (8): e675–e686. doi:10.1016/j.jogc.2018.05.033. PMID 30103892.
- Alós Cortés JI, Andreu Domingo A, Arribas Mir L, Cabero Roura L, Cueto Lopez M, López Sastre J, Melchor Marcos JC, Puertas Prieto A, de la Rosa Fraile M, Salcedo Abizanda S, Sánchez Luna M, Sánchez Pérez MJ, Torrejón Cardoso R. (2012). "Prevención de la infección perinatal por estreptococo del grupo B. Recomendaciones españolas revisadas 2012" (PDF). Revista Espanola de Quimioterapia. 25 (1): 79–88. PMID 22488547. Retrieved 25 November 2019.CS1 maint: multiple names: authors list (link)
- Surbek D.Kommission für Qualitätssicherung der SGGG/gynécologie suisse (2007). "Prophylaxe der frühen Neugeborenensepsis durch Streptokokken der Gruppe B-Prevention of early neonatal sepsis by GBS". Gynakologisch-Geburtshilfliche Rundschau. 47 (2): 103–104. doi:10.1159/000100342. PMID 17440274. S2CID 77887846.
- Leitlinien der Gesellschaft für Neonatologie und Pädiatrische Intensivmedizin (GNPI) Deutschen Gesellschaft für Gynäkologie und Geburtshilfe, Deutschen Gesellschaft für Pädiatrische Infektiologie (DGPI), und Deutsche Gesellschaft für Perinatale Medizin (DGPM). "Prophylaxe der Neugeborensepsis - frühe Form - durch Streptokokken der Gruppe B - Prevention of neonatal sepsis - early form - by GBS" (PDF). Retrieved 30 November 2019.CS1 maint: multiple names: authors list (link)
- Kotarski J, Heczko PB, Lauterbach R, Niemiec T, Leszczyńska- Gorzelak B (2008). "Rekomendacje polskiego towarzystwa ginekologicznego dotyczące wykrywania nosicielstwa paciorkowców grupy B (GBS) u kobiet w ciąży i zapobiegania zakażeniom u noworodków--Recommendations Polish Gynecological Society for the detection of carriers of GBS in pregnant women and prevent infections in newborns". Ginekol Pol. 79: 221–223.
- A. Měchurová; V. Unzeitig; J. Mašata; P. Švihovec (2013). "Diagnostika a léčba streptokoků skupiny B v těhotenství a za porodu – doporučený postup---Diagnosis and treatment of GBS in pregnancy and during birth - Recommendations" (PDF). Klin Mikrobiol Infekc Lek. 12: 11–14.
- Agence Nationale d’Accreditation et d’Evaluation en Santé (2001). "Prévention anténatale du risque infectieux bactérien néonatal précoce.2001" (PDF). Retrieved 25 November 2019.
- Belgian Health Council. "Prevention of perinatal group B streptococcal infections. Guidelines. 2003" (PDF). Retrieved 25 November 2019.
- Nederlandse Vereniging voor Obstetrie en Gynaecologie. "2008. PREVENTIE VAN NEONATALE GROEP-B-STREPTOKOKKENZIEKTE (GBS-ZIEKTE) Versie 2.0" (PDF). Retrieved 25 November 2019.
- Ministerio de Salud de la Nación. Dirección Nacional de Salud Materno Infantil. Argentina, In Spanish. "Recomendaciones para la prevención, diagnóstico y tratamiento de la infección neonatal precoz por Estreptococo β Hemolítico del Grupo B (EGB). Recommendations for prevention, diagnosis and treatment of early neonatal infection by Streptococcus β hemolytic group B (GBS)" (PDF). Retrieved 29 November 2019.
- Queensland Maternity; Neonatal Clinical Guideline. "Early onset Group B streptococcal disease" (PDF). Retrieved 29 November 2019.
- Royal Australian and New Zealand College of Obstetricians and Gynaecologists. RANZCOG. "Streptococcus (GBS) in Pregnancy: Screening and Management. July 2019.»" (PDF). Retrieved 25 November 2019.
- Raabe VN, Shane AL. (2019). Group B Streptococcus (Streptococcus agalactiae). Microbiology Spectrum. 7. pp. 228–238. doi:10.1128/microbiolspec.GPP3-0007-2018. ISBN 9781683670124. PMC 6432937. PMID 30900541.
- Farley MM. (2001). "Group B Streptococcal Disease in Nonpregnant Adults" (PDF). Clinical Infectious Diseases. 33 (4): 556–561. doi:10.1086/322696. PMID 11462195.
- Edwards MS,. Baker CJ. (2005). "Group B streptococcal infections in elderly adults" (PDF). Clinical Infectious Diseases. 41 (6): 839–847. doi:10.1086/432804. PMID 16107984.
- Skoff TH, Farley MM, Petit S, Craig AS, Schaffner W, Gershman K, Harrison LH, Lynfield R, Mohle-Boetani J, Zansky S, Albanese BA, Stefonek K, Zell ER, Jackson D, Thompson T, Schrag SJ (2009). "Increasing Burden of Invasive Group B Streptococcal Disease in Nonpregnant Adults, 1990–2007" (PDF). Clinical Infectious Diseases. 49 (1): 85–92. doi:10.1086/599369. PMID 19480572.
- Al Akhrass F, Abdallah L, Berger S, Hanna R, Reynolds N, Thompson S, Hallit R, Schlievert PM. (2013). "Streptococcus agalactiae toxic shock-like syndrome: two case reports and review of the literature". Medicine. 92 (1): 10–14. doi:10.1097/MD.0b013e31827dea11. PMC 5370747. PMID 23263717.CS1 maint: multiple names: authors list (link)
- Group B Strep Support (GBSS). "Home»Get Involved»Campaign»Group B Strep Awareness Month Group B Strep Awareness Month". Retrieved 25 November 2019.
- "Welcome".
- Jordan HT, Farley MM, Craig A, Mohle-Boetani J, Harrison LH, Petit S, Lynfield R, Thomas A, Zansky S, Gershman K, Albanese BA, Schaffner W, Schrag SJ; Active Bacterial Core Surveillance (ABCs)/Emerging Infections Program Network, CDC (2008). "Revisiting the need for vaccine prevention of late-onset neonatal group B streptococcal disease: a multistate, population-based analysis". The Pediatric Infectious Disease Journal. 27 (12): 1057–1064. doi:10.1097/inf.0b013e318180b3b9. PMID 18989238.CS1 maint: multiple names: authors list (link)
- Edwards MS, Rench MA, Rinaudo CD, Fabbrini M, Tuscano G, Buffi G, Bartolini E, Bonacci S, Baker CJ, Margarit I (2016). "Immune Responses to Invasive Group B Streptococcal Disease in Adults". Emerging Infectious Diseases. 22 (11): 1877–1883. doi:10.3201/eid2211.160914. PMC 5088039. PMID 27767008.
- "GBS vaccine research and development technical roadmap and WHO Preferred Product Characteristics". World Health Organization. Retrieved 30 November 2019.
- "Group B Streptococcus infection causes an estimated 150,000 preventable stillbirths and infant deaths every year". World Health Organization. 6 November 2017. Retrieved 30 November 2019.
- Baker CJ, Carey VJ, Rench MA, Edwards MS, Hillier SH, Kasper DL, Platt R (2014). "Maternal Antibody at Delivery Protects Neonates From Early Onset Group B Streptococcal Disease" (PDF). Journal of Infectious Diseases. 209 (5): 781–788. doi:10.1093/infdis/jit549. PMC 3923540. PMID 24133184.
- Rodriguez-Granger J, Alvargonzalez JC, Berardi A, Berner R, Kunze M, Hufnagel M, Melin P, Decheva A, Orefici G, Poyart C, Telford J, Efstratiou A, Killian M, Krizova P, Baldassarri L, Spellerberg B, Puertas A, Rosa-Fraile M (2012). "Prevention of group B streptococcal neonatal disease revisited. The DEVANI European project". European Journal of Clinical Microbiology & Infectious Diseases. 31 (9): 2097–2114. doi:10.1007/s10096-012-1559-0. PMID 22314410. S2CID 15588906.
- Edwards MS, Gonik B (2013). "Preventing the broad spectrum of perinatal morbidity and mortality throughgh group B streptococcal vaccination". Vaccine. 31S: D66–71. doi:10.1016/j.vaccine.2012.11.046. PMID 23200934.
- Madhi, Shabir A; Cutland, Clare L; Jose, Lisa; Koen, Anthonet; Govender, Niresha; Wittke, Frederick; Olugbosi, Morounfolu; Meulen, Ajoke Sobanjo-ter; Baker, Sherryl; Dull, Peter M; Narasimhan, Vas; Slobod, Karen (2016). "Safety and immunogenicity of an investigational maternal trivalent group B streptococcus vaccine in healthy women and their infants: a randomised phase 1b/2 trial". The Lancet Infectious Diseases. 16 (8): 923–934. doi:10.1016/S1473-3099(16)00152-3. ISSN 1473-3099. PMID 27139805.
- Heath PT. (2016). "Status of vaccine research and development of vaccines for GBS". Vaccine. 34 (26): 2876–2879. doi:10.1016/j.vaccine.2015.12.072. PMID 26988258.
- Song JY, Lim JH, Lim S, Yong Z, Seo HS (2018). "Progress toward a group B streptococcal vaccine". Human Vaccines & Immunotherapeutics. 14 (11): 2669–2681. doi:10.1080/21645515.2018.1493326. PMC 6314413. PMID 29995578.CS1 maint: multiple names: authors list (link)
- Ria, Francesco; Gupalova, T.; Leontieva, G.; Kramskaya, T.; Grabovskaya, K.; Bormotova, E.; Korjevski, D.; Suvorov, A. (2018). "Development of experimental GBS vaccine for mucosal immunization". PLOS ONE. 13 (5): e0196564. Bibcode:2018PLoSO..1396564G. doi:10.1371/journal.pone.0196564. ISSN 1932-6203. PMC 5935385. PMID 29727446.
- Nuccitelli A, Rinaudo CD, Maione D (2015). "Group B Streptococcus vaccine: state of the art". Therapeutic Advances in Vaccines. 3 (3): 76–90. doi:10.1177/2051013615579869. PMC 4530403. PMID 26288735.
- Davies HG, Carreras-Abad C, Le Doare K, Heath PT. (2019). "Group B Streptococcus: Trials and Tribulations" (PDF). The Pediatric Infectious Disease Journal. 38(6S Suppl 1.) (6S Suppl 1): S72-76. doi:10.1097/INF.0000000000002328. PMID 31205250.CS1 maint: multiple names: authors list (link)
- Delannoy CM, Crumlish M, Fontaine MC, Pollock J, Foster G, Dagleish MP, Turnbull JF, Zadoks RN (2013). "Human Streptococcus agalactiae strains in aquatic mammal and fish". BMC Microbiology. 13: 41. doi:10.1186/1471-2180-13-41. PMC 3585737. PMID 23419028.
- Ruegg PL (2017). "A 100-Year Review: Mastitis detection, management, and prevention". Journal of Dairy Science. 100 (12): 10381–10397. doi:10.3168/jds.2017-13023. PMID 29153171. Retrieved 29 November 2019.
- Keefe GP (1997). "Streptococcus agalactiae mastitis: a review". Can. Vet. J. 38: 429–37. PMC 1576741. PMID 9220132.
- Evans JJ, Klesius PH, Pasnik DJ, Bohnsack JF (2009). "Human Streptococcus agalactiae isolate in Nile tilapia (Oreochromis niloticus)". Emerging Infectious Diseases. 15 (5): 774–776. doi:10.3201/eid1505.080222. PMC 2687030. PMID 19402966.
- Liu G, Zhang W, Lu C (2013). "Comparative genomics analysis of Streptococcus". BMC Genomics. 14: 775. doi:10.1186/1471-2164-14-775. PMC 3831827. PMID 24215651.
- Li LP, Wang R, Liang WW1, Huang T1, Huang Y2, Luo FG, Lei AY, Chen M, Gan X (2015). "Development of live attenuated Streptococcus agalactiae vaccine for tilapia via continuous passage in vitro". Fish & Shellfish Immunology. 45 (2): 955–963. doi:10.1016/j.fsi.2015.06.014. PMID 26087276.CS1 maint: multiple names: authors list (link)
- Zhang D, Gao Y, Li Q, Ke X, Liu Z, Lu M, Shi C. (2019). "An effective live attenuated vaccine against Streptococcus agalactiae infection in farmed Nile tilapia (Oreochromis niloticus)". Fish & Shellfish Immunology. S1050-4648: 31087–3. doi:10.1016/j.fsi.2019.11.044. PMID 31751658.CS1 maint: multiple names: authors list (link)
External links
- http://gbss.org.uk/ Group B Strep Association] UK Group B Strep Association] UK
- CDC—Group B Strep (GBS)
| Classification | |
|---|---|
| External resources |