Etonogestrel birth control implant
Etonogestrel birth control implant, sold under the brand name Nexplanon among others, is a device made up of a single rod containing etonogestrel which is used for birth control.[5] It is one of the most effective forms of birth control with a one-year failure rate around 0.05%.[6] It lasts at least three or four years with some data showing effectiveness for five years.[2][4][7][8] The device is placed under the skin.[2] Following removal fertility quickly returns.[5]
| Etonogestrel birth control implant | |
|---|---|
 Implanon | |
| Background | |
| Type | Hormonal Progestin-only implant |
| First use | 1998 |
| Synonyms | Etonogestrel contraceptive implant |
| Trade names | Implanon, Nexplanon, others |
| AHFS/Drugs.com | FDA Professional Drug Information |
| Failure rates (first year) | |
| Perfect use | 0.05%[1] |
| Typical use | 0.05%[1] |
| Usage | |
| Duration effect | 3 to 5 years[2][3] |
| Reversibility | Yes |
| User reminders | Requires removal after the 3–5 years[4] |
| Advantages and disadvantages | |
| STI protection | No |
| Weight | May cause weight gain |
| Period disadvantages | May cause irregular or prolonged bleeding |
| Period advantages | Minimizes pain. In 33% no periods. |
| Benefits | Long-term contraception. |
Common side effects include menstrual changes including irregular bleeding, with approximately one third of women reporting no menstrual periods.[5][9][10] It is not recommended in people with liver disease.[10] The etonogestrel implant is a type of long-acting reversible birth control.[6] It works by stopping ovulation, thickening the mucus around the opening of the cervix, and altering the lining of the uterus.[11]
Etonogestrel implants were approved for medical use in Indonesia in 1998 and in the United States in 2006.[9][12] It is on the World Health Organization's List of Essential Medicines.[13] It is available as a generic medication.[14] Etonogestrel implants are approved in more than 90 countries and used by about three million women globally as of 2010.[11][12]
Medical uses
Etonogestrel birth control implants are a type of long-acting reversible contraception, which has been shown to be one of the most effective form of birth control.[15] The failure rate of the implants is 0.05% for both perfect use and typical use because the method requires no user action after placement.[16] Studies of one type, which include over 2,467 women-years of exposure, found no pregnancies.[17][18][19]
Other studies have found some failures with this method, some attributed to failures of the method itself and others to improper placement, drug interactions, or conception prior to method insertion.[20]
In comparison, tubal sterilization has a failure rate of 0.5% and IUDs have a failure rate of 0.2–0.8%.[16] A single implant is approved for three years with data showing effectiveness for five years.[7][4]
Side effects
Irregular bleeding and spotting: Many women will experience some type of irregular, unpredictable, prolonged, frequent, or infrequent bleeding.[21] Some women also experience amenorrhea. For some women, prolonged bleeding will decline after the first three months of use. However, other women may experience this bleeding pattern through all five years of use. While these patterns are not dangerous, they are the most common reason that women give for discontinuing the use of the implant. After removal, bleeding patterns return to previous patterns in most women.[17][18][19]
Insertion complications: Some minor side effects such as bruising, skin irritation, or pain around the insertion site are common.[17] However, there are some rare complications that can occur, such as infection or expulsion.[17][22] In some cases, a serious complication occurs when the provider fails to insert, and the rod is left in the inserter. An Australian study reported 84 pregnancies as a result of such failure.[20]
Migration: Although very rare, the rod can sometimes move slightly within the arm. This can make removal more difficult. It is possible that insertion in the same site as a previous implant increases the likelihood of migration.[22] Rods can be located only through high-frequency ultrasound or magnetic resonance imaging (MRI).[17] It can be located using traditional X-ray or CT-scan because of the inclusion of barium sulphate. There have been rare reports of implants having reached the lung via the pulmonary artery.[23] Correct subdermal insertion reduces the risk of these events.
Possible weight gain: Some women may experience slight weight gain when using the implant.[17] However, current studies are not conclusive because they do not compare the weight of women using implants with a control group of women not using the implant. The average increase in body weight in studies was less than 5 pounds (2,25 kg) over 2 years.[18]
Ovarian cysts: A small portion of women using implants and other contraceptive implants develop ovarian cysts.[17] Usually these cysts will disappear without treatment.[24]
Drug interactions: Efavirenz appears to decrease etonogestrel levels[25] and increase rates of undesired pregnancy among implant users.
Pregnancy: it is recommended that implants be removed if a pregnancy does occur. However, there is no evidence to suggest that the implant has a negative effect on pregnancy or a developing fetus.[17]
Acne: Acne has been self-reported to be a side effect, and is listed as a side effect by the FDA. However, a study of users found that a majority of users with acne before their insertion reported that their acne had decreased, and only 16% of those who did not have acne before insertion developed acne.[18]
Other possible symptoms: Other symptoms that have been reported in trials of implants include headache, emotional lability, abdominal pain, loss of libido, and vaginal dryness.[17] However, there have been no studies that conclusively determine that these symptoms are caused by the implant.[18][19]
Contraindications
Women should not use implants if they:[26]
- Are, or think they are, pregnant
- Are allergic to etonogestrel
- Have vaginal bleeding that has not been explained
- Have some forms of severe liver disease
A full list of contraindications can be found in the WHO Medical Eligibility Criteria for Contraceptive Use 2015 and the CDC United States Medical Eligibility Criteria for Contraceptive Use 2016
Device description
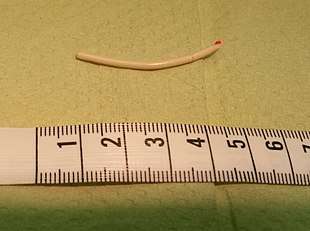
Nexplanon/Implanon consists of a single rod made of ethylene vinylacetate copolymer that is 4 cm long and 2mm in diameter.[21] It is similar to a matchstick in size. The rod contains 68 mg of etonogestrel (sometimes called 3-keto-destrogestrel), a type of progestin.[17] Peak serum etonogestrel concentrations have been found to reach 781–894 pg/mL in the first few weeks, gradually decreasing to 192–261 pg/mL after 1 year, 154–194 pg/mL after 2 years, and 156–177 pg/mL after 3 years, maintaining ovulation suppression and contraceptive efficacy.[27] Serum levels maintain relatively stable through 36 months, which implies that the method may be effective for longer than 3 years.[28]
Although not formally approved by the manufacturer for more than 3 years, studies have shown it remains a highly effective contraceptive for 5 years.[7]
It is a type of progestogen-only contraception.
Insertion and removal
An experienced clinician must perform the insertion of implants to ensure proper insertion and minimize the risk of nerve damage or misplacement, which could result in pregnancy.[29] Before insertion, the arm is washed with a cleaning solution and a local anesthetic is applied to the upper arm around the insertion area.[17] A needle-like applicator is used to insert the rod under the skin into the subdermal tissue on the inner side of the arm posterior to the groove between the biceps and triceps muscles.[30] The average time for insertion is 0.5 to 1 minute.[18][19] A bandage should be kept on the insertion site for 24 hours afterwards. Bruising and mild discomfort are common after insertion.[17] Serious insertion site complications such as infection can occur very rarely, in less than 1% of patients. If a woman receives an implant outside the first five days of her period, she should wait to have sex or use a backup method of contraception (such as a condom, female condom, diaphragm, sponge, or emergency contraception) for the following week after insertion to prevent pregnancy. However, if the implant is inserted during the first five days of a woman's period, she is protected for that cycle and beyond.[31]
Implants can be removed at any time if pregnancy is desired. The rod must also be removed by an experienced clinician. At removal, a local anesthetic is again used around the implant area at the distal end.[17] If the provider cannot feel the implant, imaging tests may be necessary to locate the rod before it can be removed. A small incision is made in the skin over the end of the implant site. In some cases, a fibrous sheath may have formed around the implant, in which case the sheath must be incised.[17] The implant is removed using forceps. The removal procedure lasts, on average, 3 to 3.5 minutes.[18][19]
Mechanism of action
The mechanism of action of progestin only contraceptives depends on the progestin activity and dose.[33] Intermediate dose progestin-only contraceptives, like Nexplanon or Implanon (and the progestin-only pill Cerazette) allow some follicular development but inhibit ovulation in almost all cycles as the primary mechanism of action. Ovulation was not observed in studies of Implanon in the first two years of use and only rarely in the third year with no pregnancies. A secondary mechanism of action is the progestogenic increase in cervical mucus viscosity which inhibits sperm penetration.[34] Hormonal contraceptives also have effects on the endometrium that theoretically could affect implantation, however no scientific evidence indicates that prevention of implantation actually results from their use.[35]
History
The possibility of the subdermal contraceptive implant began when silicone was discovered in the 1940s and found to be bio-compatible with the human body.[36] In 1964, Folkman and Long published the first study demonstrating that such a rod could be used to deliver drugs.[37] In 1966 Dziuk and Cook published a study that looked at release rates and suggested that the rods could be well suited for contraception.[38] After a study that used implants with progestogens for contraception, the Population Council developed and patented Norplant and Jadelle.[39] Norplant has six rods and is considered a first-generation implant. Jadelle (Norplant II), a two-rod implant, and other single rod implants that followed, were developed because of complications resulting from Norplant's 6-rod system. The Jadelle system contains two silicone rods mixed with levonorgestrel. In 1990 De Nijs patented a co-axial extrusion technique of ethylene vinylacetate copolymers and 3-keto-desogestrel (etonogestrel) for the preparation of long-acting contraceptive devices, such as Implanon, Nexplanon and Nuvaring.[40] The single rods were less visible under the skin and used etonogestrel as opposed to levonorgestrel in the hopes that it would reduce side effects.[36]
Norplant was used internationally beginning in 1983 and was marketed in the United States and the United Kingdom in 1993. There were many complications associated with Norplant removal in the United States and it was taken off the market in 2002. Although Jadelle was approved by the FDA, it has never been marketed in the United States, but it is widely used in Africa and Asia.[39] Implanon was first used in Indonesia in 1998 and approved for use in the United States in 2006. Nexplanon was developed to eliminate the problem of non-insertion and localization of Implanon by changing the inserter device and making the rod radiopaque.[41] As of January 2012, Implanon is no longer being marketed and Nexplanon is the only available single-rod implant.
Types
Nexplanon and Implanon NXT are essentially identical to Implanon except Nexplanon and Implanon NXT have 15 mg of barium sulphate added to the core, so it is detectable by x-ray.[41][7] Nexplanon and Implanon NXT also has a pre-loaded applicator for easier insertion.[42]
References
- Trussell J (2011). "Contraceptive efficacy" (PDF). In Hatcher RA, Trussell J, Nelson AL, Cates W, Kowal D, Policar MS (eds.). Contraceptive technology (20th revised ed.). New York: Ardent Media. pp. 779–863. ISBN 978-1-59708-004-0. ISSN 0091-9721. OCLC 781956734. Archived (PDF) from the original on 2013-11-12.
- Hamilton RJ (2016). Tarascon Pocket Pharmacopoeia 2016 Deluxe Lab-Coat Edition. Jones & Bartlett Publishers. p. 392. ISBN 9781284095289.
- Melville C (2015). Sexual and Reproductive Health at a Glance. John Wiley & Sons. p. 21. ISBN 9781118460757.
- Lotke, Pamela S. (2016). Contraception, An Issue of Obstetrics and Gynecology Clinics, E-Book. Elsevier Health Sciences. p. 634. ISBN 9780323402590.
- World Health Organization (2015). The selection and use of essential medicines. Twentieth report of the WHO Expert Committee 2015 (including 19th WHO Model List of Essential Medicines and 5th WHO Model List of Essential Medicines for Children). Geneva: World Health Organization. pp. 332–36. hdl:10665/189763. ISBN 9789241209946. ISSN 0512-3054. WHO technical report series;994.
- Wipf J (2015). Women's Health, An Issue of Medical Clinics of North America. Elsevier Health Sciences. pp. 507–509. ISBN 9780323376082. Archived from the original on 2017-09-24.
- Hatcher, Robert Anthony (September 2018). Contraceptive technology. Hatcher, Robert A. (Robert Anthony), 1937- (21st ed.). New York, NY. pp. Chapter 4, specifically pages 129–134. ISBN 978-1732055605. OCLC 1048947218.
- "Long-Acting Reversible Contraception: Implants and Intrauterine Devices - ACOG". www.acog.org. Retrieved 20 August 2019.
- Shoupe D, Mishell DR (2015). The Handbook of Contraception: A Guide for Practical Management (2 ed.). Humana Press. p. 140. ISBN 9783319201856. Archived from the original on 2017-09-24.
- "Implanon - FDA prescribing information, side effects and uses". www.drugs.com. Archived from the original on 1 January 2017. Retrieved 1 January 2017.
- Pattman R, Sankar N, Handy P, Price DA (2010). Oxford Handbook of Genitourinary Medicine, HIV, and Sexual Health. OUP Oxford. p. 368. ISBN 9780199571666. Archived from the original on 2017-09-24.
- Senanayake P, Potts M (2008). Atlas of Contraception, Second Edition (2 ed.). CRC Press. p. 53. ISBN 9780203347324. Archived from the original on 2017-09-24.
- World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. p. 791. ISBN 9780857113382.
- Winner B, Peipert JF, Zhao Q, Buckel C, Madden T, Allsworth JE, Secura GM (May 2012). "Effectiveness of long-acting reversible contraception". The New England Journal of Medicine. 366 (21): 1998–2007. doi:10.1056/nejmoa1110855. PMID 22621627.
- Guttmacher (2012). "Contraceptive Use in the United States". Archived from the original on 2016-12-11.
- Raymond EG (2011). "Contraceptive Implants". In Hatcher RA, Nelson TJ, Guest F, Kowal D (eds.). Contraceptive technology (19th revised ed.). New York: Ardent Media. pp. 144–156.
- Funk S, Miller MM, Mishell DR, Archer DF, Poindexter A, Schmidt J, Zampaglione E (May 2005). "Safety and efficacy of Implanon, a single-rod implantable contraceptive containing etonogestrel". Contraception. 71 (5): 319–26. doi:10.1016/j.contraception.2004.11.007. PMID 15854630.
- Flores JB, Balderas ML, Bonilla MC, Vázquez-Estrada L (September 2005). "Clinical experience and acceptability of the etonogestrel subdermal contraceptive implant". International Journal of Gynaecology and Obstetrics. 90 (3): 228–33. doi:10.1016/j.ijgo.2005.06.007. PMID 16043175.
- Harrison-Woolrych M, Hill R (April 2005). "Unintended pregnancies with the etonogestrel implant (Implanon): a case series from postmarketing experience in Australia". Contraception. 71 (4): 306–8. doi:10.1016/j.contraception.2004.10.005. PMID 15792651.
- Adams K, Beal MW (2009). "Implanon: a review of the literature with recommendations for clinical management". Journal of Midwifery & Women's Health. 54 (2): 142–9. doi:10.1016/j.jmwh.2008.09.004. PMID 19249660.
- Smith A, Reuter S (October 2002). "An assessment of the use of Implanon in three community services". The Journal of Family Planning and Reproductive Health Care. 28 (4): 193–6. doi:10.1783/147118902101196540. PMID 12419059.
- "Nexplanon (etonogestrel) contraceptive implants: Reports of device in vasculature and lung". Archived from the original on 2016-09-18. Retrieved 2016-07-31.
- Brache V, Faundes A, Alvarez F, Cochon L (January 2002). "Nonmenstrual adverse events during use of implantable contraceptives for women: data from clinical trials". Contraception. 65 (1): 63–74. doi:10.1016/s0010-7824(01)00289-x. PMID 11861056.
- Vieira, Carolina S.; Bahamondes, Maria V.; de Souza, Roberto M.; Brito, Milena B.; Rocha Prandini, Tatiana R.; Amaral, Eliana; Bahamondes, Luis; Duarte, Geraldo; Quintana, Silvana M. (2014-08-01). "Effect of antiretroviral therapy including lopinavir/ritonavir or efavirenz on etonogestrel-releasing implant pharmacokinetics in HIV-positive women". Journal of Acquired Immune Deficiency Syndromes. 66 (4): 378–385. doi:10.1097/QAI.0000000000000189. ISSN 1944-7884. PMID 24798768.
- "US CDC Medical Eligibility Criteria for Contraceptive Use". 2016.
- "Implanon label" (PDF). FDA. 2010-10-26. Archived from the original on 2010-03-10. Retrieved 2010-10-26.
- Mäkäräinen L, van Beek A, Tuomivaara L, Asplund B, Coelingh Bennink H (April 1998). "Ovarian function during the use of a single contraceptive implant: Implanon compared with Norplant". Fertility and Sterility. 69 (4): 714–21. doi:10.1016/s0015-0282(98)00015-6. PMID 9548163.
- Wechselberger G, Wolfram D, Pülzl P, Soelder E, Schoeller T (July 2006). "Nerve injury caused by removal of an implantable hormonal contraceptive". American Journal of Obstetrics and Gynecology. 195 (1): 323–6. doi:10.1016/j.ajog.2005.09.016. PMID 16813761.
- "Nexplanon Prescribing Information" (PDF). Retrieved 18 August 2020.
- Bedsider (2010). "Implant." Retrieved from http://bedsider.org/methods/implant#how_to_tab Archived 2013-07-28 at the Wayback Machine on March 17, 2011.
- Davies GC, Feng LX, Newton JR, Van Beek A, Coelingh-Bennink HJ (March 1993). "Release characteristics, ovarian activity and menstrual bleeding pattern with a single contraceptive implant releasing 3-ketodesogestrel". Contraception. 47 (3): 251–61. doi:10.1016/0010-7824(93)90042-6. PMID 8462316.
- Glasier A (2006). "Contraception". In DeGroot LJ, Jameson JL (eds.). Endocrinology (5th ed.). Philadelphia: Elsevier Saunders. pp. 3000–1. ISBN 978-0-7216-0376-6.
- Organon (April 2006). "Implanon SPC (Summary of Product Characteristics)". Archived from the original on 2007-09-30. Retrieved 2007-04-15.
- Rivera R, Yacobson I, Grimes D (November 1999). "The mechanism of action of hormonal contraceptives and intrauterine contraceptive devices". American Journal of Obstetrics and Gynecology. 181 (5 Pt 1): 1263–9. doi:10.1016/S0002-9378(99)70120-1. PMID 10561657.
- Ladipo OA, Akinso SA (April 2005). "Contraceptive implants". African Journal of Reproductive Health. 9 (1): 16–23. doi:10.2307/3583156. JSTOR 3583156. PMID 16104651.
- Folkman J, Long DM (March 1964). "The use of silicone rubber as a carrier for prolonged drug therapy". The Journal of Surgical Research. 4 (3): 139–42. doi:10.1016/s0022-4804(64)80040-8. PMID 14130164.
- Dziuk PJ, Cook B (January 1966). "Passage of steroids through silicone rubber". Endocrinology. 78 (1): 208–11. doi:10.1210/endo-78-1-208. PMID 5948426.
- Association of Reproductive Health Professionals (July 2008). "The Single-Rod Contraceptive Implant". Archived from the original on 2018-03-20.
- US granted 4957119, De Nijs H, "Contraceptive Implant", issued 18 September 1990, assigned to Akzo NV
- Mansour D (October 2010). "Nexplanon(®): what Implanon(®) did next". The Journal of Family Planning and Reproductive Health Care. 36 (4): 187–9. doi:10.1783/147118910793048629. PMID 21067632.
- Ormsby A (5 Jan 2011). "Contraceptive alert after women fall pregnant". Reuters. Archived from the original on 10 May 2011. Retrieved 10 May 2011.