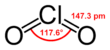
Chlorine dioxide
Chlorine dioxide is a chemical compound with the formula ClO2 that exists as yellowish-green gas above 11 °C, a reddish-brown liquid between −59 °C and 11 °C, and as bright orange crystals when colder. It is an oxidizing agent, able to transfer oxygen to a variety of substrates, while gaining one or more electrons via oxidation-reduction (redox). It does not hydrolyze when it enters water, and is usually handled as a dissolved gas in solution in water. Potential hazards with chlorine dioxide include health concerns, explosiveness and fire ignition.[4] It is commonly used as a bleach.
| |||
 | |||
| Names | |||
|---|---|---|---|
| IUPAC name
Chlorine dioxide | |||
| Other names
Chlorine(IV) oxide | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.030.135 | ||
| EC Number |
| ||
| E number | E926 (glazing agents, ...) | ||
| 1265 | |||
| MeSH | Chlorine+dioxide | ||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 9191 | ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| ClO2 | |||
| Molar mass | 67.45 g·mol−1 | ||
| Appearance | Yellow to reddish gas | ||
| Odor | Acrid | ||
| Density | 2.757 g dm−3[1] | ||
| Melting point | −59 °C (−74 °F; 214 K) | ||
| Boiling point | 11 °C (52 °F; 284 K) | ||
| 8 g/L (at 20 °C) | |||
| Solubility | soluble in alkaline and sulfuric acid solutions | ||
| Vapor pressure | >1 atm[2] | ||
Henry's law constant (kH) |
4.01×10−2 atm m3 mol−1 | ||
| Acidity (pKa) | 3.0(5) | ||
| Thermochemistry | |||
Std molar entropy (S |
257.22 J K−1 mol−1 | ||
Std enthalpy of formation (ΔfH⦵298) |
104.60 kJ/mol | ||
| Hazards | |||
| Main hazards | Acute toxicity | ||
| Safety data sheet | Safety Data Sheet Archive. | ||
| GHS pictograms |    | ||
| GHS Signal word | Danger | ||
GHS hazard statements |
H271, H314, H330 | ||
| P210, P220, P280, P283, P260, P264, P271, P284, P301, P330, P331, P311, P306, P360, P304, P340, P305, P351, P338, P371+380+375, P405, P403+233, P501 | |||
| NFPA 704 (fire diamond) | |||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose) |
292 mg/kg (oral, rat)[3] | ||
LCLo (lowest published) |
260 ppm (rat, 2 hr)[3] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible) |
TWA 0.1 ppm (0.3 mg/m3)[2] | ||
REL (Recommended) |
TWA 0.1 ppm (0.3 mg/m3) ST 0.3 ppm (0.9 mg/m3)[2] | ||
IDLH (Immediate danger) |
5 ppm[2] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Chlorine dioxide was discovered in 1811 and has been widely used for bleaching purposes in the paper industry, and for treatment of drinking water. More recent developments have extended its application into food processing, disinfection of premises and vehicles, mold eradication, air disinfection and odor control, treatment of swimming pools, dental applications, and wound cleansing.
The compound has been fraudulently marketed as an ingestible cure for a wide range of diseases, including childhood autism[5] and COVID-19.[6][7][8] Children who have been given enemas of chlorine dioxide as a supposed cure for childhood autism have suffered life-threatening ailments.[5] The U.S. Food and Drug Administration (FDA) has stated that ingestion or other internal use of chlorine dioxide (other than perhaps oral rinsing under dentist supervision) has no health benefits and it should not be used internally for any reason.[9][10]
Structure and bonding
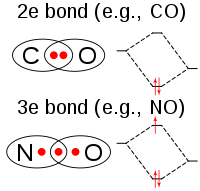
Chlorine dioxide is a neutral chlorine compound. It is very different from elemental chlorine, both in its chemical structure and in its behavior.[11] One of the most important qualities of chlorine dioxide is its high water solubility, especially in cold water. Chlorine dioxide does not hydrolyze when it enters water; it remains a dissolved gas in solution. Chlorine dioxide is approximately 10 times more soluble in water than chlorine.[11]
The molecule ClO2 has an odd number of valence electrons, and therefore, it is a paramagnetic radical. Its electronic structure has long baffled chemists because none of the possible Lewis structures is very satisfactory. In 1933, L. O. Brockway proposed a structure that involved a three-electron bond.[12] Chemist Linus Pauling further developed this idea and arrived at two resonance structures involving a double bond on one side and a single bond plus three-electron bond on the other.[13] In Pauling's view the latter combination should represent a bond that is slightly weaker than the double bond. In molecular orbital theory this idea is commonplace if the third electron is placed in an anti-bonding orbital. Later work has confirmed that the highest occupied molecular orbital is indeed an incompletely-filled antibonding orbital.[14]
Preparation
Chlorine dioxide is a compound that can decompose extremely violently when separated from diluting substances. As a result, preparation methods that involve producing solutions of it without going through a gas-phase stage are often preferred. Arranging handling in a safe manner is essential.
Oxidation of chlorite
In the laboratory, ClO2 can be prepared by oxidation of sodium chlorite with chlorine:[15]
- 2 NaClO2 + Cl2 → 2 ClO2 + 2 NaCl
Traditionally, chlorine dioxide for disinfection applications has been made from sodium chlorite or the sodium chlorite–hypochlorite method:
- 2 NaClO2 + 2 HCl + NaOCl → 2 ClO2 + 3 NaCl + H2O
or the sodium chlorite–hydrochloric acid method:
- 5 NaClO2 + 4 HCl → 5 NaCl + 4 ClO2 + 2 H2O
or the chlorite–sulfuric acid method:
- 4 ClO−
2 + 2 H2SO4 → 2 ClO2 + HClO3 + 2 SO2−
4 + H2O + HCl
All three methods can produce chlorine dioxide with high chlorite conversion yield. Unlike the other processes, the chlorite–sulfuric acid method produces completely chlorine-free chlorine dioxide, although it suffers from the requirement of 25% more chlorite to produce an equivalent amount of chlorine dioxide. Alternatively, hydrogen peroxide may be efficiently used in small-scale applications.[11]
Reduction of chlorate
In the laboratory, chlorine dioxide can also be prepared by reaction of potassium chlorate with oxalic acid:
- 2 KClO3 + 2 H2C2O4 → K2C2O4 + 2 ClO2 + 2 CO2 + 2 H2O
- 2 KClO3 + H2C2O4 + 2 H2SO4 → 2 KHSO4 + 2 ClO2 + 2 CO2 + 2 H2O
Over 95% of the chlorine dioxide produced in the world today is made by reduction of sodium chlorate, for use in pulp bleaching. It is produced with high efficiency in a strong acid solution with a suitable reducing agent such as methanol, hydrogen peroxide, hydrochloric acid or sulfur dioxide.[11] Modern technologies are based on methanol or hydrogen peroxide, as these chemistries allow the best economy and do not co-produce elemental chlorine. The overall reaction can be written as:[16]
- chlorate + acid + reducing agent → chlorine dioxide + by-products
As a typical example, the reaction of sodium chlorate with hydrochloric acid in a single reactor is believed to proceed through the following pathway:
- ClO−
3 + Cl−
+ H+
→ ClO−
2 + HOCl - ClO−
3 + ClO−
2 + 2 H+
→ 2 ClO
2 + H
2O - HOCl + Cl−
+ H+
→ Cl
2 + H
2O
which gives the overall reaction
- 2 ClO−
3 + 2 Cl−
+ 4 H+
→ 2 ClO
2 + Cl
2 + 2 H
2O.
The commercially more important production route uses methanol as the reducing agent and sulfuric acid for the acidity. Two advantages of not using the chloride-based processes are that there is no formation of elemental chlorine, and that sodium sulfate, a valuable chemical for the pulp mill, is a side-product. These methanol-based processes provide high efficiency and can be made very safe.[11]
The variant process using chlorate, hydrogen peroxide and sulfuric acid has been increasingly used since 1999 for water treatment and other small-scale disinfection applications, since it produce a chlorine-free product at high efficiency.
Other processes
Very pure chlorine dioxide can also be produced by electrolysis of a chlorite solution:[17]
- 2 NaClO2 + 2 H2O → 2 ClO2 + 2 NaOH + H2
High-purity chlorine dioxide gas (7.7% in air or nitrogen) can be produced by the gas–solid method, which reacts dilute chlorine gas with solid sodium chlorite:[17]
- 2 NaClO2 + Cl2 → 2 ClO2 + 2 NaCl
Handling properties
At partial pressures above 10 kPa[11] (or gas-phase concentrations greater than 10% volume in air at STP), ClO2 may explosively decompose into chlorine and oxygen. The decomposition can be initiated by light, hot spots, chemical reaction, or pressure shock. Thus, chlorine dioxide gas is never handled in concentrated form, but is almost always handled as a dissolved gas in water in a concentration range of 0.5 to 10 grams per liter. Its solubility increases at lower temperatures, thus it is common to use chilled water (5 °C) when storing at concentrations above 3 grams per liter. In many countries, such as the United States, chlorine dioxide gas may not be transported at any concentration and is almost always produced at the application site using a chlorine dioxide generator.[11] In some countries, chlorine dioxide solutions below 3 grams per liter in concentration may be transported by land, however, they are relatively unstable and deteriorate quickly.
Uses
Chlorine dioxide is used for bleaching of wood pulp and for the disinfection (called chlorination) of municipal drinking water.[18][19]:4–1[20] As a disinfectant, it is effective even at low concentrations because of its unique qualities.[11][19]
Bleaching
Chlorine dioxide is sometimes used for bleaching of wood pulp in combination with chlorine, but it is used alone in ECF (elemental chlorine-free) bleaching sequences. It is used at moderately acidic pH (3.5 to 6). The use of chlorine dioxide minimizes the amount of organochlorine compounds produced.[21] Chlorine dioxide (ECF technology) currently is the most important bleaching method worldwide. About 95% of all bleached kraft pulp is made using chlorine dioxide in ECF bleaching sequences.[22]
Water treatment
The Niagara Falls, New York, water treatment plant first used chlorine dioxide for drinking water treatment in 1944 for destroying "taste and odor producing phenolic compounds".[19]:4–17[20] Chlorine dioxide was introduced as a drinking water disinfectant on a large scale in 1956, when Brussels, Belgium, changed from chlorine to chlorine dioxide.[20] Its most common use in water treatment is as a pre-oxidant prior to chlorination of drinking water to destroy natural water impurities that would otherwise produce trihalomethanes on exposure to free chlorine.[24][25][26] Trihalomethanes are suspect carcinogenic disinfection by-products[27] associated with chlorination of naturally occurring organics in the raw water.[26] Chlorine dioxide is also superior to chlorine when operating above pH 7,[19]:4–33 in the presence of ammonia and amines and for the control of biofilms in water distribution systems.[26] Chlorine dioxide is used in many industrial water treatment applications as a biocide including cooling towers, process water, and food processing.[28]
Chlorine dioxide is less corrosive than chlorine and superior for the control of Legionella bacteria.[20][29] Chlorine dioxide is superior to some other secondary water disinfection methods in that chlorine dioxide is an EPA-registered biocide, is not negatively impacted by pH, does not lose efficacy over time (the bacteria will not grow resistant to it), and is not negatively impacted by silica and phosphates, which are commonly used potable water corrosion inhibitors.
It is more effective as a disinfectant than chlorine in most circumstances against waterborne pathogenic agents such as viruses,[30] bacteria and protozoa – including the cysts of Giardia and the oocysts of Cryptosporidium.[19]:4–20–4–21
The use of chlorine dioxide in water treatment leads to the formation of the by-product chlorite, which is currently limited to a maximum of 1 part per million in drinking water in the USA.[19]:4–33 This EPA standard limits the use of chlorine dioxide in the US to relatively high-quality water because this minimizes chlorite concentration, or water that is to be treated with iron-based coagulants (iron can reduce chlorite to chloride).
Use in public crises
Chlorine dioxide has many applications as an oxidizer or disinfectant.[11] Chlorine dioxide can be used for air disinfection[31] and was the principal agent used in the decontamination of buildings in the United States after the 2001 anthrax attacks.[32] After the disaster of Hurricane Katrina in New Orleans, Louisiana, and the surrounding Gulf Coast, chlorine dioxide was used to eradicate dangerous mold from houses inundated by the flood water.[33]
In addressing the COVID-19 pandemic, the U.S. Environmental Protection Agency has posted a list of many disinfectants that meet its criteria for use in environmental measures against the causative coronavirus.[34][35] Some are based on sodium chlorite that is activated into chlorine dioxide, though differing formulations are used in each product. Many other products on the EPA list contain sodium hypochlorite, which is similar in name but should not be confused with sodium chlorite because they have very different modes of chemical action.
Other disinfection uses
Chlorine dioxide may be used as a fumigant treatment to "sanitize" fruits such as blueberries, raspberries, and strawberries that develop molds and yeast.[36]
Chlorine dioxide may be used to disinfect poultry by spraying or immersing it after slaughtering.[37]
Chlorine dioxide may be used for the disinfection of endoscopes, such as under the trade name Tristel.[38] It is also available in a trio consisting of a preceding pre-clean with surfactant and a succeeding rinse with deionized water and a low-level antioxidant.[39]
Chlorine dioxide may be used for control of zebra and quagga mussels in water intakes.[19]:4–34
Chlorine dioxide was shown to be effective in bedbug eradication.[40]
Pseudomedicine
Chlorine dioxide is fraudulently marketed as a magic cure for a range of diseases from brain cancer to AIDS. Enemas of chlorine dioxide are a supposed cure for childhood autism, resulting in complaints to the FDA reporting life-threatening reactions,[41] and even death.[42] Chlorine dioxide is relabelled to a variety of brand names including, but not limited to MMS, Miracle Mineral Solution and CD protocol.[43] There is no scientific basis for chlorine dioxide's medical properties and FDA has warned against its usage.[44][45]
Other uses
Chlorine dioxide is used as an oxidant for destroying phenols in wastewater streams and for odor control in the air scrubbers of animal byproduct (rendering) plants.[19]:4–34 It is also available for use as a deodorant for cars and boats, in chlorine dioxide-generating packages that are activated by water and left in the boat or car overnight.
Safety issues in water and supplements
Chlorine dioxide is toxic, hence limits on exposure to it are needed to ensure its safe use. The United States Environmental Protection Agency has set a maximum level of 0.8 mg/L for chlorine dioxide in drinking water.[46] The Occupational Safety and Health Administration (OSHA), an agency of the United States Department of Labor, has set an 8-hour permissible exposure limit of 0.1 ppm in air (0.3 mg/m3) for people working with chlorine dioxide.[47]
On July 30, 2010, and again on October 1, 2010, the United States Food and Drug Administration warned against the use of the product "Miracle Mineral Supplement", or "MMS", which when made up according to instructions produces chlorine dioxide. MMS has been marketed as a treatment for a variety of conditions, including HIV, cancer, autism, and acne. The FDA warnings informed consumers that MMS can cause serious harm to health and stated that it has received numerous reports of nausea, diarrhea, severe vomiting, and life-threatening low blood pressure caused by dehydration.[48][49] This warning was repeated for a third time on 12 August 2019, and a fourth on April 8, 2020, stating that ingesting MMS is the same as drinking bleach, and urging consumers to not use them or give these products to their children for any reason.[45]
References
- Haynes, William M. (2010). Handbook of Chemistry and Physics (91 ed.). Boca Raton, Florida: CRC Press. p. 4–58. ISBN 978-1439820773.
- NIOSH Pocket Guide to Chemical Hazards. "#0116". National Institute for Occupational Safety and Health (NIOSH).
- "Chlorine dioxide". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- "Toxicological Profile for Chlorine Dioxide and Chlorite" (PDF). Agency for Toxic Substances and Disease Registry, US HHS. Archived from the original (PDF) on 2019-06-14.
- "Parents are poisoning their children with bleach to 'cure' autism. These moms are trying to stop it". NBC News. Retrieved 2019-05-21.
- "Fake news: Chlorine dioxide won't stop coronavirus". Detroit News. Retrieved 2020-04-03.
- Friedman, Lisa (2020-04-03). "E.P.A. Threatens Legal Action Against Sellers of Fake Coronavirus Cleaners". The New York Times. ISSN 0362-4331. Retrieved 2020-04-03.
- Spencer, Sarnac Hale. "Those coronavirus 'cures' you're hearing about? They're fake. Don't drink chlorine dioxide". USA TODAY. Retrieved 2020-04-03.
- "Drinking bleach will not cure cancer or autism, FDA warns". NBC News. Retrieved 2019-08-13.
- Food and Drug Administration (2019-08-12). "FDA warns consumers about the dangerous and potentially life threatening side effects of Miracle Mineral Solution". fda.gov. Archived from the original on 2019-08-14. Retrieved 2019-08-16.
- Vogt, H.; Balej, J.; Bennett, J. E.; Wintzer, P.; Sheikh, S. A.; Gallone, P.; Vasudevan, S.; Pelin, K. "Chlorine Oxides and Chlorine Oxygen Acids". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH.
- Brockway, L. O. (March 1933). "The Three-Electron Bond in Chlorine Dioxide" (PDF). Proceedings of the National Academy of Sciences. 19 (3): 303–307. Bibcode:1933PNAS...19..303B. doi:10.1073/pnas.19.3.303. PMC 1085967. PMID 16577512.
- Pauling, Linus (1988). General chemistry. Mineola, NY: Dover Publications. ISBN 0-486-65622-5.
- Flesch, R.; Plenge, J.; Rühl, E. (2006). "Core-level excitation and fragmentation of chlorine dioxide". International Journal of Mass Spectrometry. 249-250: 68–76. Bibcode:2006IJMSp.249...68F. doi:10.1016/j.ijms.2005.12.046.
- Derby, R. I.; Hutchinson, W. S. (1953). Chlorine(IV) Oxide. Inorganic Syntheses. 4. pp. 152–158. doi:10.1002/9780470132357.ch51. ISBN 978-0-470-13235-7.
- Ni, Y.; Wang, X. (1996). "Mechanism of the Methanol Based ClO2 Generation Process". International Pulp Bleaching Conference. TAPPI. pp. 454–462.
- White, George W.; White, Geo Clifford (1999). The handbook of chlorination and alternative disinfectants (4th ed.). New York: John Wiley. ISBN 0-471-29207-9.
- Swaddle, Thomas Wilson (1997). Inorganic Chemistry: An Industrial and Environmental Perspective. Academic Press. pp. 198–199. ISBN 0-12-678550-3.
- Alternative Disinfectants and Oxidants Manual, chapter 4: Chlorine Dioxide (PDF), US Environmental Protection Agency: Office of Water, April 1999, archived from the original on 2015-09-05, retrieved 2009-11-27CS1 maint: unfit url (link)
- Block, Seymour Stanton (2001). Disinfection, Sterilization, and Preservation (5th ed.). Lippincott, Williams & Wilkins. p. 215. ISBN 0-683-30740-1.
- Sjöström, E. (1993). Wood Chemistry: Fundamentals and Applications. Academic Press. ISBN 0-12-647480-X. OCLC 58509724.
- "AET – Reports – Science – Trends in World Bleached Chemical Pulp Production: 1990–2005". Archived from the original on 2017-07-30. Retrieved 2016-02-26.
- Harrel, C. G. (1952). "Maturing and Bleaching Agents in Producing Flour". Industrial & Engineering Chemistry. 44 (1): 95–100. doi:10.1021/ie50505a030.
- Sorlini, S.; Collivignarelli, C. (2005). "Trihalomethane formation during chemical oxidation with chlorine, chlorine dioxide and ozone of ten Italian natural waters". Desalination. 176 (1–3): 103–111. doi:10.1016/j.desal.2004.10.022.
- Li, J.; Yu, Z.; Gao, M. (1996). "A pilot study on trihalomethane formation in water treated by chlorine dioxide". Zhonghua Yufang Yixue Zazhi (Chinese Journal of Preventive Medicine) (in Chinese). 30 (1): 10–13. PMID 8758861.
- Volk, C. J.; Hofmann, R.; Chauret, C.; Gagnon, G. A.; Ranger, G.; Andrews, R. C. (2002). "Implementation of chlorine dioxide disinfection: Effects of the treatment change on drinking water quality in a full-scale distribution system". Journal of Environmental Engineering and Science. 1 (5): 323–330. doi:10.1139/s02-026.
- Pereira, M. A.; Lin, L. H.; Lippitt, J. M.; Herren, S. L. (1982). "Trihalomethanes as initiators and promoters of carcinogenesis". Environmental Health Perspectives. 46: 151–156. doi:10.2307/3429432. JSTOR 3429432. PMC 1569022. PMID 7151756.
- Andrews, L.; Key, A.; Martin, R.; Grodner, R.; Park, D. (2002). "Chlorine dioxide wash of shrimp and crawfish an alternative to aqueous chlorine". Food Microbiology. 19 (4): 261–267. doi:10.1006/fmic.2002.0493.
- Zhang, Zhe; McCann, Carole; Stout, Janet E.; Piesczynski, Steve; Hawks, Robert; Vidic, Radisav; Yu, Victor L. (2007). "Safety and Efficacy of Chlorine Dioxide for Legionella control in a Hospital Water System" (PDF). Infection Control and Hospital Epidemiology. 28 (8): 1009–1012. doi:10.1086/518847. PMID 17620253. Retrieved 2009-11-27.
- Ogata, N.; Shibata, T. (January 2008). "Protective effect of low-concentration chlorine dioxide gas against influenza A virus infection". Journal of General Virology. 89 (pt 1): 60–67. doi:10.1099/vir.0.83393-0. PMID 18089729.
- Zhang, Y.-L.; Zheng, S.-Y.; Zhi, Q. (2007). "Air Disinfection with Chlorine Dioxide in Saps". Journal of Environment and Health. 24 (4): 245–246.
- "Anthrax spore decontamination using chlorine dioxide". United States Environmental Protection Agency. 2007. Retrieved 2009-11-27.
- Sy, Kaye V.; McWatters, Kay H.; Beuchat, Larry R. (2005). "Efficacy of Gaseous Chlorine Dioxide as a Sanitizer for Killing Salmonella, Yeasts, and Molds on Blueberries, Strawberries, and Raspberries". Journal of Food Protection. International Association for Food Protection. 68 (6): 1165–1175. doi:10.4315/0362-028x-68.6.1165. PMID 15954703.
- "How we know disinfectants should kill the COVID-19 coronavirus". Chemical & Engineering News. Retrieved 2020-03-28.
- US EPA, OCSPP (2020-03-13). "List N: Disinfectants for Use Against SARS-CoV-2". US EPA. Retrieved 2020-03-28.
- O'Brian, D. (2017). "Chlorine Dioxide Pouches Can Make Produce Safer and Reduce Spoilage". AgResearch Magazine. USDA Agricultural Research Service (July). Retrieved 2018-06-21.
- "The truth behind the chlorinated chicken panic". The Big Issue. 2019-05-29. Retrieved 2020-02-05.
- Coates, D. (2001). "An evaluation of the use of chlorine dioxide (Tristel One-Shot) in an automated washer/disinfector (Medivator) fitted with a chlorine dioxide generator for decontamination of flexible endoscopes". Journal of Hospital Infection. 48 (1): 55–65. doi:10.1053/jhin.2001.0956. PMID 11358471.
- "Tristel Wipes System Product Information" (PDF). Ethical Agents. Archived from the original (PDF) on 2016-04-15. Retrieved 2012-11-01.
- Gibbs, S. G.; Lowe, J. J.; Smith, P. W.; Hewlett, A. L. (2012). "Gaseous chlorine dioxide as an alternative for bedbug control". Infection Control & Hospital Epidemiology. 33 (5): 495–9. doi:10.1086/665320. PMID 22476276. S2CID 14105046.
- Bartley, Lisa (2016-10-29). "Group of SoCal parents secretly try to cure kids with autism using bleach". ABC 7 News. ABC. Retrieved 2019-03-24.
- Ryan, Frances (2016-07-13). "The fake cures for autism that can prove deadly". The Guardian. Guardian Media Group. Retrieved 2019-03-24.
- "The Parents Who Give Their Children Bleach Enemas to 'Cure' Them of Autism". vice.com. 2015-03-12. Retrieved 2018-04-05.
- "FDA Warns Consumers of Serious Harm from Drinking Miracle Mineral Solution (MMS)". 2011-02-03. Archived from the original on 2011-02-03. Retrieved 2018-04-05.
- Food and Drug Administration (2019-08-12). "FDA warns consumers about the dangerous and potentially life threatening side effects of Miracle Mineral Solution". fda.gov. Archived from the original on 2019-08-14. Retrieved 2019-08-16.
- "ATSDR: ToxFAQs™ for Chlorine Dioxide and Chlorite".
- "Occupational Safety and Health Guideline for Chlorine Dioxide". Archived from the original on 2012-12-04. Retrieved 2012-12-08.
- "Press Announcements – FDA Warns Consumers of Serious Harm from Drinking Miracle Mineral Solution (MMS)". Archived from the original on 2017-01-12.
- "'Miracle' Treatment Turns into Potent Bleach". U.S. Food and Drug Administration. 2015-11-20. Archived from the original on 2017-11-01. Retrieved 2017-12-06.