Nickel(II) oxide
Nickel(II) oxide is the chemical compound with the formula NiO. It is the principal oxide of nickel.[3] It is classified as a basic metal oxide. Several million kilograms are produced annually of varying quality, mainly as an intermediate in the production of nickel alloys.[4]The mineralogical form of NiO, bunsenite, is very rare. Other nickel(III) oxides have been claimed, for example: Ni
2O
3 and NiO
2, but they have yet to be proven by X-ray crystallography.[3]
 | |
| Names | |
|---|---|
| IUPAC name
Nickel(II) oxide | |
| Other names
Nickel monoxide Oxonickel | |
| Identifiers | |
| ECHA InfoCard | 100.013.833 |
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| Properties | |
| NiO | |
| Molar mass | 74.6928 g/mol |
| Appearance | green crystalline solid |
| Density | 6.67 g/cm3 |
| Melting point | 1,955 °C (3,551 °F; 2,228 K) |
| negligible | |
| Solubility | soluble in KCN |
| +660.0·10−6 cm3/mol | |
Refractive index (nD) |
2.1818 |
| Thermochemistry | |
Std enthalpy of formation (ΔfH⦵298) |
-240.0 kJ/mol |
| Hazards | |
| Safety data sheet | JT Baker |
EU classification (DSD) (outdated) |
Carc. Cat. 1 Toxic (T) |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
LDLo (lowest published) |
5000 mg/kg (rat, oral)[2] |
| Related compounds | |
Other anions |
Nickel(II) selenide Nickel(II) telluride |
Other cations |
Palladium(II) oxide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Production
NiO can be prepared by multiple methods. Upon heating above 400 °C, nickel powder reacts with oxygen to give NiO. In some commercial processes, green nickel oxide is made by heating a mixture of nickel powder and water at 1000 °C, the rate for this reaction can be increased by the addition of NiO.[5] The simplest and most successful method of preparation is through pyrolysis of a nickel(II) compounds such as the hydroxide, nitrate, and carbonate, which yield a light green powder.[3] Synthesis from the elements by heating the metal in oxygen can yield grey to black powders which indicates nonstoichiometry.[3]
Structure
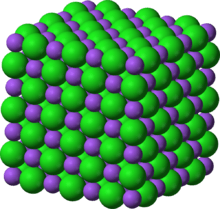
NiO adopts the NaCl structure, with octahedral Ni2+ and O2− sites. The conceptually simple structure is commonly known as the rock salt structure. Like many other binary metal oxides, NiO is often non-stoichiometric, meaning that the Ni:O ratio deviates from 1:1. In nickel oxide, this non-stoichiometry is accompanied by a color change, with the stoichiometrically correct NiO being green and the non-stoichiometric NiO being black.
Applications and reactions
NiO has a variety of specialized applications and generally applications distinguish between "chemical grade", which is relatively pure material for specialty applications, and "metallurgical grade", which is mainly used for the production of alloys. It is used in the ceramic industry to make frits, ferrites, and porcelain glazes. The sintered oxide is used to produce nickel steel alloys. Charles Édouard Guillaume won the 1920 Nobel Prize in Physics for his work on nickel steel alloys which he called invar and elinvar.
NiO was also a component in the nickel-iron battery, also known as the Edison Battery, and is a component in fuel cells. It is the precursor to many nickel salts, for use as specialty chemicals and catalysts. More recently, NiO was used to make the NiCd rechargeable batteries found in many electronic devices until the development of the environmentally superior NiMH battery.[5] NiO an anodic electrochromic material, have been widely studied as counter electrodes with tungsten oxide, cathodic electrochromic material, in complementary electrochromic devices.
About 4000 tons of chemical grade NiO are produced annually.[4] Black NiO is the precursor to nickel salts, which arise by treatment with mineral acids. NiO is a versatile hydrogenation catalyst.
Heating nickel oxide with either hydrogen, carbon, or carbon monoxide reduces it to metallic nickel. It combines with the oxides of sodium and potassium at high temperatures (>700 °C) to form the corresponding nickelate.[5]
Electronic structure
NiO is useful for illustrating the failure of density functional theory (using functionals based on the local-density approximation) and Hartree–Fock theory to account for the strong correlation. The term strong correlation refers to behavior of electrons in solids that is not well described (often not even in a qualitatively correct manner) by simple one-electron theories such as the local-density approximation (LDA) or Hartree–Fock theory.[6] For instance, the seemingly simple material NiO has a partially filled 3d-band (the Ni atom has 8 of 10 possible 3d-electrons) and therefore would be expected to be a good conductor. However, strong Coulomb repulsion (a correlation effect) between d-electrons makes NiO instead a wide band gap Mott insulator. Thus, strongly correlated materials have electronic structures that are neither simply free-electron-like nor completely ionic, but a mixture of both.[7][8]
Health risks
Long-term inhalation of NiO is damaging to the lungs, causing lesions and in some cases cancer.[9]
The calculated half-life of dissolution of NiO in blood is more than 90 days.[10] NiO has a long retention half-time in the lungs; after administration to rodents, it persisted in the lungs for more than 3 months.[11][10] Nickel oxide is classified as a human carcinogen[12][13][14][15][16][17] based on increased respiratory cancer risks observed in epidemiological studies of sulfidic ore refinery workers.[18]
In a 2-year National Toxicology Program green NiO inhalation study, some evidence of carcinogenicity in F344/N rats but equivocal evidence in female B6C3F1 mice was observed; there was no evidence of carcinogenicity in male B6C3F1 mice.[12] Chronic inflammation without fibrosis was observed in the 2-year studies.
References
- "Safety Data Sheet" (PDF). Northwest Missouri State University.
- "Nickel metal and other compounds (as Ni)". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- Greenwood, Norman N.; Earnshaw, Alan (1984). Chemistry of the Elements. Oxford: Pergamon Press. pp. 1336–37. ISBN 978-0-08-022057-4.
- Kerfoot, Derek G. E. (2000). "Nickel". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a17_157.
- "Handbook of Inorganic Chemicals", Pradniak, Pradyot; McGraw-Hill Publications,2002
- Hüfner, S. (1994-04-01). "Electronic structure of NiO and related 3d-transition-metal compounds". Advances in Physics. 43 (2): 183–356. Bibcode:1994AdPhy..43..183H. doi:10.1080/00018739400101495. ISSN 0001-8732.
- Kuiper, P.; Kruizinga, G.; Ghijsen, J.; Sawatzky, G. A.; Verweij, H. (1989). "Character of Holes in LixNi1−xO and Their Magnetic Behavior". Physical Review Letters. 62 (2): 221–224. Bibcode:1989PhRvL..62..221K. doi:10.1103/physrevlett.62.221. ISSN 0031-9007. PMID 10039954.
- Mott, N. F. (1949). "The Basis of the Electron Theory of Metals, with Special Reference to the Transition Metals". Proceedings of the Physical Society. Section A. 62 (7): 416–422. Bibcode:1949PPSA...62..416M. doi:10.1088/0370-1298/62/7/303. ISSN 0370-1298.
- "Toxicology and Carcinogenesis Studies of Nickel Oxide", U.S. Dept. of Health and Human Services, No. 451, 07/1996
- English, J.C., Parker, R.D.R., Sharma, R.P. & Oberg, S.G. (1981). Toxicokinetics of nickel in rats after intratracheal administration of a soluble and insoluble form. Am Ind Hyg Assoc J. 42(7):486-492.
- Benson, J.M., Barr, E.B., Bechtold, W.E., Cheng, Y-S., Dunnick, J.K., Eastin, W.E., Hobbs, C.H., Kennedy, C.H. & Maples, K.R. (1994). Fate of inhaled nickel oxide and nickel subsulfie in F344/N rats. Inhal Toxicol 6(2):167-183.
- National Toxicology Program (NTP) (1996). Toxicology and Carcinogenesis Studies of Nickel Oxide (CAS No. 1313-99-1) in F344 Rats and B6C3F1 Mice (inhalation studies) US DHHS. NTP TR 451. NIH Publication No.96-3367.
- Sunderman, F.W., Hopfer, S.M., Knight, J.A., Mccully, K.S., Cecutti, A.G., Thornhill, P.G., Conway, K., Miller, C., Patierno, S.R. & Costa, M. (1987). Physicochemical characteristics and biological effects of nickel oxides. Carcinogenesis 8(2):305-313.
- IARC (2012). “Nickel and nickel compounds” IARC Monogr Eval Carcinog Risks Hum, Volume 100C: 169-218. (https://monographs.iarc.fr/ENG/Monographs/vol100C/mono100C-10.pdf).
- Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on Classification, Labelling and Packaging of Substances and Mixtures, Amending and Repealing Directives 67/548/EEC and 1999/45/EC and amending Regulation (EC) No 1907/2006 [OJ L 353, 31.12.2008, p. 1]. Annex VI. www.eur-lex.europa.eu/legal-content/en/TXT/?uri=CELEX%3A32008R1272 Accessed July 13, 2017.
- Globally Harmonised System of Classification and Labelling of Chemicals (GHS), Fifth revised edition, United Nations, New York and Geneva, 2013. PDF unece.org Accessed July 13, 2017.
- NTP (National Toxicology Program). 2016. “Report on Carcinogens”, 14th Edition.; Research Triangle Park, NC: U.S. Department of Health and Human Services, Public Health Service. https://ntp.niehs.nih.gov/pubhealth/roc/index-1.html Accessed July 13, 2017.
- International Committee on Nickel Carcinogenesis in Man (ICNCM). (1990). Report of the International Committee on Nickel Carcinogenesis in Man. Scan. J. Work Environ. Health. 16(1): 1-82.
External links
- Bunsenite at mindat.org
- Bunsenite mineral data
