Potassium persulfate
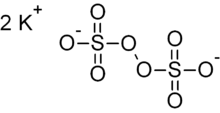
Potassium persulfate is the inorganic compound with the formula K2S2O8. Also known as potassium peroxydisulfate or KPS, it is a white solid that is sparingly soluble in cold water, but dissolves better in warm water. This salt is a powerful oxidant, commonly used to initiate polymerizations.
 | |
 | |
| Names | |
|---|---|
| Other names
potassium peroxydisulfate Anthion potassium perdisulfate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.028.893 |
| EC Number |
|
| E number | E922 (glazing agents, ...) |
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 1492 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| K2S2O8 | |
| Molar mass | 270.322 g/mol |
| Appearance | white powder |
| Odor | odorless |
| Density | 2.477 g/cm3[1] |
| Melting point | < 100 °C (212 °F; 373 K) (decomposes) |
| 1.75 g/100 mL (0 °C) 4.49 g/100 ml (20 °C) | |
| Solubility | insoluble in alcohol |
Refractive index (nD) |
1.467 |
| Structure | |
| triclinic | |
| Hazards | |
| Safety data sheet | ICSC 1133 |
| GHS pictograms | 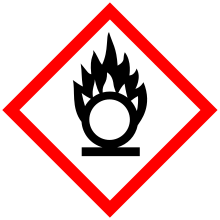   |
| GHS Signal word | Danger |
GHS hazard statements |
H272, H302, H315, H317, H319, H334, H335, H371 |
| P220, P261, P280, P305+351+338, P342+311 | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
802 mg/kg (oral, rat)[2] |
| Related compounds | |
Other anions |
Potassium sulfite Potassium sulfate Potassium peroxymonosulfate |
Other cations |
Sodium persulfate Ammonium persulfate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation
Potassium persulfate can be prepared by electrolysis of a cold solution potassium bisulfate in sulfuric acid at a high current density.[1]
- 2 KHSO4 → K2S2O8 + H2
It can also be prepared by adding potassium bisulfate (KHSO4) to a solution of the more soluble salt ammonium peroxydisulfate (NH4)2S2O8. In principle it can be prepared by chemical oxidation of potassium sulfate using fluorine.
Uses
This salt is used to initiate polymerization of various alkenes leading to commercially important polymers such as styrene-butadiene rubber and polytetrafluoroethylene and related materials. In solution, the dianion dissociates to give radicals:[3]
- [O3SO-OSO3]2− ⇌ 2 [SO4]•−
It is used in organic chemistry as an oxidizing agent,[4] for instance in the Elbs persulfate oxidation of phenols and the Boyland–Sims oxidation of anilines.
As a strong yet stable bleaching agent it also finds use in various hair bleaches and lighteners. Such brief and non-continuous use is normally hazard free, however prolonged contact can cause skin irritation.[5] It has been used as an improving agent for flour with the E number E922, although it is no longer approved for this use within the EU.
Precautions
The salt is a strong oxidant and is incompatible with organic compounds. Prolonged skin contact can result in irritation.[5]
References
- Brauer, Georg (1963). Handbook of Preparative Inorganic Chemistry. 1 (2nd ed.). New York: Academic Press. p. 392. ISBN 978-0121266011.
- Chambers, Michael. "ChemIDplus - 7727-21-1 - USHAGKDGDHPEEY-UHFFFAOYSA-L - Potassium persulfate". chem.nlm.nih.gov.
- Harald Jakob; Stefan Leininger; Thomas Lehmann; Sylvia Jacobi; Sven Gutewort. "Peroxo Compounds, Inorganic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a19_177.pub2.
- Encyclopedia of Reagents for Organic Synthesis, vol. 1, pp. 193–197(1995)
- Pang, S; Fiume, MZ (January 2001). "Final Report on the Safety Assessment of Ammonium, Potassium, and Sodium Persulfate". International Journal of Toxicology. 20 (3): 7-21. doi:10.1080/10915810152630710. PMID 11766134.
