Chromium(II) oxide
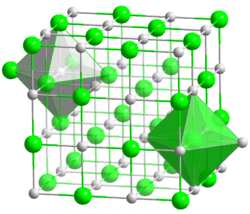
Chromium(II) oxide (CrO) is an inorganic compound composed of chromium and oxygen.[1] It is a black powder that crystallises in the rock salt structure.[2] Hypophosphites may reduce chromium(III) oxide to chromium(II) oxide:
- H3PO2 + 2 Cr2O3 → 4 CrO + H3PO4
 | |
| Names | |
|---|---|
| IUPAC name
chromium(II) oxide | |
| Identifiers | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| Properties | |
| CrO | |
| Molar mass | 67.996 g/mol |
| Appearance | black |
| Melting point | 300 °C (572 °F; 573 K) (decomposes) |
| Structure | |
| cubic, cF8 | |
| Fm3m, No. 225 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
It is readily oxidized by the atmosphere. CrO is basic, while CrO
3 is acidic, and Cr
2O
3 is amphoteric.[3]
See also
- Chromium(IV) oxide
- Chromium(VI) oxide
References
- Satish. Anand, Raj. Kumar (1989), Dictionary of Inorganic Chemistry, Anmol Publications, ISBN 978-81-7041-236-6
- Egon Wiberg, Arnold Frederick Holleman (2001) Inorganic Chemistry, Elsevier ISBN 0-12-352651-5
- Chemistry 7th edition, by Raymond Chang page 645 (problem 15.100)
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.