Ammonium dinitramide
Ammonium dinitramide (ADN) is the ammonium salt of dinitraminic acid. ADN decomposes under heat to leave only nitrogen, oxygen, and water. The ions are the ammonium ion NH4+ and the dinitramide N(NO2)2−.
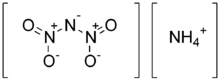 | |
| Names | |
|---|---|
| IUPAC name
Ammonium dinitramide | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.126.585 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| H4N4O4 | |
| Molar mass | 124.06 g/mol |
| Density | 1.81 g/cm3 |
| Melting point | 93 °C (199 °F; 366 K) |
| Boiling point | decomposes at 127 °C (261 °F; 400 K) |
| Hazards | |
| GHS pictograms |     |
| GHS Signal word | Danger |
GHS hazard statements |
H201, H228, H302, H371 |
| P210, P230, P240, P241, P250, P260, P264, P270, P280, P301+312, P309+311, P330, P370+378, P370+380, P372, P373, P401, P405, P501 | |
| Thermochemistry | |
Gibbs free energy (ΔfG˚) |
−150.6 kJ/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
It makes an excellent solid rocket oxidizer with a slightly higher specific impulse than ammonium perchlorate and more importantly, does not leave corrosive hydrogen chloride fumes. This property is also of military interest because halogen free smoke is harder to detect. It decomposes into low molecular mass gases so it contributes to higher performance without creating excessive temperatures if used in gun or rocket propellants. The salt is prone to detonation under high temperatures and shock more so than the perchlorate.
The EURENCO Bofors company produced LMP-103S as a 1-to-1 substitute for hydrazine by dissolving 65% ammonium dinitramide, NH4N(NO2)2, in 35% water solution of methanol and ammonia. LMP-103S has 6% higher specific impulse and 30% higher impulse density than hydrazine monopropellant. Additionally, hydrazine is highly toxic and carcinogenic, while LMP-103S is only moderately toxic. LMP-103S is UN Class 1.4S allowing for transport on commercial aircraft, and was demonstrated on the Prisma satellite in 2010. Special handling is not required. LMP-103S could replace hydrazine as the most commonly used monopropellant.[1]
The ADN-based monopropellant FLP-106 is reported to have improved properties relative to LMP-103S, including higher performance (ISP of 259 s vs. 252 s) and density (1.362 g/cm3 vs. 1.240 g/cm3).[2]
History
Ammonium dinitramide was invented in 1971 in Zelinskiy Institute of Organic Chemistry in the USSR. Initially all information related to this compound was classified because of its use as a rocket propellant, particularly in Topol-M intercontinental ballistic missiles. In 1989 ammonium dinitramide was independently synthesized at SRI International.[3] SRI obtained US and international patents for ADN in the mid-1990s, at which time scientists from the former Soviet Union revealed they had discovered ADN 18 years earlier.[3]
Preparation
There are at least 20 different synthesis routes that produce Ammonium dinitramide In the laboratory ammonium dinitramide, can be prepared by nitration of sulfamic acid or its salt under low temperatures. KO3S-NH2 + 2HNO3 W KHSO4 + NH4N(NO2)2 + H2O The process is performed under red light, since the compound is decomposed by higher energy photons. The details of the synthesis remain classified. Other sources report ammonium synthesis from ammonium nitrate, anhydrous nitric acid, and fuming sulfuric acid containing 20% free sulfur trioxide. A base other than ammonia must be added before the acid dinitramide decomposes. The final product is obtained by fractional crystallization. Another synthesis known as the urethane synthesis method requires four synthesis steps and results in a yield of up to 60 %. The processing method is not difficult and the amount of waste produced is relatively low. The urethane can be cleaned and recycled within the process.
C2H5O2C-NHNO2 + NH3 W C2H5O2C-NNO2NH4 C2H5O2C-NNO2NH4 + N2O5 W C2H5O2C-N(NO2)2 + NH4NO3 C2H5O2C-N(NO2)2 + 2NH3 W C2H5O2C-NH2 + NH4N(NO2)2
References
- Swedish Space Corporation Group, Monopropellant LMP-103S, 2011, www.ecap.se
- Anders Larsson; Niklas Wingborg. "Green Propellants Based on Ammonium Dinitramide (ADN)" (PDF). Retrieved 21 July 2020.
- "Dinitramide Salts: ADN Plus Other Salts". SRI International. Archived from the original on 2012-05-26. Retrieved 2012-04-15.
Further reading
- Modern rocket fuels> PDF> Hesiserman Online Library
- Textbook of Chemistry 1999 Prentice Press, New York
- Subbiah Venkatachalam; Gopalakrishnan Santhosh; Kovoor Ninan Ninan (2004). "An Overview on the Synthetic Routes and Properties of Ammonium Dinitramide (ADN) and other Dinitramide Salts". Propellants, Explosives, Pyrotechnics. 29 (3): 178–187. doi:10.1002/prep.200400043.
|https://application.wiley-vch.de/books/sample/3527302409_c01.pdf }}