Cicada
The cicadas (/sɪˈkɑːdə/ or /sɪˈkeɪdə/) are a superfamily, the Cicadoidea, of insects in the order Hemiptera (true bugs). They are in the suborder Auchenorrhyncha,[lower-alpha 1] along with smaller jumping bugs such as leafhoppers and froghoppers. The superfamily is divided into two families, Tettigarctidae, with two species in Australia, and Cicadidae, with more than 3,000 species described from around the world; many species remain undescribed.
| Cicada | |
|---|---|
 | |
| Annual cicada, Neotibicen linnei | |
| Calling song of Magicicada cassinii | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Hemiptera |
| Infraorder: | Cicadomorpha |
| Superfamily: | Cicadoidea Latreille, 1802 |
| Families | |
Cicadas have prominent eyes set wide apart, short antennae, and membranous front wings. They have an exceptionally loud song, produced in most species by the rapid buckling and unbuckling of drumlike tymbals. The earliest known fossil Cicadomorpha appeared in the Upper Permian period; extant species occur all around the world in temperate to tropical climates. They typically live in trees, feeding on watery sap from xylem tissue and laying their eggs in a slit in the bark. Most cicadas are cryptic. The vast majority of species are active during the day as adults, with some calling at dawn or dusk. Only a rare few species are known to be nocturnal.
One genus, the periodical cicadas spend most of their lives as underground nymphs, emerging only after 13 or 17 years. The unusual duration and timing of their emergence may reduce the number of cicadas lost to predation, both by making them a less reliably available prey (so that any predator who evolved to depend on cicadas for sustenance might starve waiting for their emergence), and by emerging in such huge numbers that they will satiate any remaining predators before losing enough of their number to threaten their survival as a species.
The annual cicadas are species that emerge every year. Though these cicadas have lifecycles that can vary from one to nine or more years as underground larvae, their emergence above ground as adults is not synchronized, so some members of each species appear every year.[1]
Cicadas have been featured in literature since the time of Homer's Iliad, and as motifs in art from the Chinese Shang dynasty. They have also been used in myth and folklore as symbols of carefree living and immortality. The cicada is also mentioned in Hesiod's Shield (ll.393–394), its voice sings when millet first ripens. Cicadas are eaten by human beings in various countries, including China, where the nymphs are served deep-fried in Shandong cuisine.
Etymology
| Look up cicada in Wiktionary, the free dictionary. |
The name is directly from the onomatopoeic Latin cicada.[2][3][lower-alpha 2]
Taxonomy and diversity
The superfamily Cicadoidea is a sister of the Cercopoidea (the froghoppers). Cicadas are arranged into two families: the Tettigarctidae and Cicadidae. The two extant species of the Tettigarctidae include one in southern Australia and the other in Tasmania. The family Cicadidae is subdivided into the subfamilies Cicadinae, Tibicininae (or Tettigadinae), Tettigomyiinae, and Cicadettinae;[4][5] they are found on all continents except Antarctica. Some previous works also included a family-level taxon called the Tibiceninae. The largest species is the Malaysian emperor cicada Megapomponia imperatoria; its wingspan is up to about 20 cm (8 in).[6] Cicadas are also notable for the great length of time some species take to mature.[7]
At least 3000 cicada species are distributed worldwide with the majority being in the tropics. Most genera are restricted to a single biogeographical region and many species have a very limited range. This high degree of endemism has been used to study the biogeography of complex island groups such as in Indonesia and Asia.[9] There are several hundred described species in Australia and New Zealand,[lower-alpha 3] around 150 in South Africa, over 170 in America north of Mexico,[10] at least 800 in Latin America,[11] and over 200 in Southeast Asia and the Western Pacific.[12] About 100 species occur in the Palaearctic. A few species are found in southern Europe,[7] and a single species was known from England, the New Forest cicada, Cicadetta montana, which also occurs in continental Europe.[13] Many species await formal description and many well-known species are yet to be studied carefully using modern acoustic analysis tools that allow their songs to be characterized.
Many of the North American species are the annual or jarfly or dog-day cicadas, members of the Neotibicen, Megatibicen, or Hadoa genera, so named because they emerge in late July and August.[14] The best-known North American genus, however, may be Magicicada. These periodical cicadas have an extremely long lifecycle of 13 or 17 years, with adults suddenly and briefly emerging in large numbers.[14][15]
Australian cicadas are found on tropical islands and cold coastal beaches around Tasmania, in tropical wetlands, high and low deserts, alpine areas of New South Wales and Victoria, large cities including Sydney, Melbourne, and Brisbane, and Tasmanian highlands and snowfields. Many of them have common names such as cherry nose, brown baker, red eye, greengrocer, yellow Monday, whisky drinker, double drummer, and black prince. The Australian greengrocer, Cyclochila australasiae, is among the loudest insects in the world.[16]

More than forty species from five genera populate New Zealand, ranging from sea level to mountain tops, and all are endemic to New Zealand and its surrounding islands (the Kermadec Islands, the Chatham Islands). One species is found on Norfolk Island which technically is part of Australia[17] The closest relatives of the NZ cicadas live in New Caledonia and Australia.

Palaeontology
Fossil Cicadomorpha first appeared in the Late Triassic. The superfamily Palaeontinoidea contains three families. The Upper Permian Dunstaniidae are found in Australia and South Africa, and also in younger rocks from China. The Upper Triassic Mesogereonidae are found in Australia and South Africa.[18] However, this group is currently thought to be more distantly related to Cicadomorpha than previously thought.[19]
The Palaeontinidae or "giant cicadas" come from the Jurassic and Lower Cretaceous of Eurasia and South America.[18] The first of these was a fore wing discovered in the Taynton Limestone Formation of Oxfordshire, England; it was initially described as a butterfly in 1873, before being recognised as a cicada like form and renamed Palaeontina oolitica.[20]
Most fossil Cicadidae are known from the Cenozoic,[21] and the oldest unambiguously identified specimen is Davispia bearcreekensis (subfamily Tibicininae) from 59–56 Ma. One fossil genus and species (Burmacicada protera) based on a first-instar nymph has recently been reported from 98–99M years ago in the Late Cretaceous,[22] although questions remain about its assignment to the Cicadidae.[21]
Biology
Description

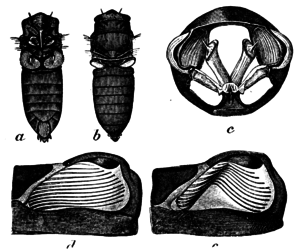
Cicadas are large insects made conspicuous by the courtship calls of the males. They are characterized by having three joints in their tarsi, and having small antennae with conical bases and three to six segments, including a seta at the tip.[23] The Auchenorrhyncha differ from other hemipterans by having a rostrum that arises from the posteroventral part of the head, complex sound-producing membranes, and a mechanism for linking the wings that involves a down-rolled edging on the rear of the fore wing and an upwardly protruding flap on the hindwing. Cicadas are feeble jumpers, and nymphs lack the ability to jump altogether. Another defining characteristic is the adaptations of the fore limbs of nymphs for underground life. The relict family Tettigarctidae differs from the Cicadidae in having the prothorax extending as far as the scutellum, and by lacking the tympanal apparatus.[9]
The adult insect, known as an imago, is 2 to 5 cm (1 to 2 in) in total length in most species, although the largest, the empress cicada (Megapomponia imperatoria), has a head-body length around 7 cm (2.8 in), and its wingspan is 18–20 cm (7–8 in).[7][24] Cicadas have prominent compound eyes set wide apart on the sides of the head. The short antennae protrude between the eyes or in front of them. They also have three small ocelli located on the top of the head in a triangle between the two large eyes; this distinguishes cicadas from other members of the Hemiptera. The mouthparts form a long, sharp rostrum that they insert into the plant to feed.[25] The postclypeus is a large, nose-like structure that lies between the eyes and makes up most of the front of the head; it contains the pumping musculature.[26]
The thorax has three segments and houses the powerful wing muscles. They have two pairs of membranous wings that may be hyaline, cloudy, or pigmented. The wing venation varies between species and may help in identification. The middle thoracic segment has an operculum on the underside, which may extend posteriorly and obscure parts of the abdomen. The abdomen is segmented, with the hindermost segments housing the reproductive organs, and terminates in females with a large, saw-edged ovipositor. In males, the abdomen is largely hollow and used as a resonating chamber.[25]
The surface of the fore wing is superhydrophobic; it is covered with minute, waxy cones, blunt spikes that create a water-repellent film. Rain rolls across the surface, removing dirt in the process. In the absence of rain, dew condenses on the wings. When the droplets coalesce, they leap several millimetres into the air, which also serves to clean the wings.[27] Bacteria landing on the wing surface are not repelled; rather, their membranes are torn apart by the nanoscale-sized spikes, making the wing surface the first-known biomaterial that can kill bacteria.[28]
Temperature regulation
Desert cicadas such as Diceroprocta apache are unusual among insects in controlling their temperature by evaporative cooling, analogous to sweating in mammals. When their temperature rises above about 39 °C, they suck excess sap from the food plants and extrude the excess water through pores in the tergum at a modest cost in energy. Such a rapid loss of water can be sustained only by feeding on water-rich xylem sap. At lower temperatures, feeding cicadas would normally need to excrete the excess water. By evaporative cooling, desert cicadas can reduce their bodily temperature by some 5 °C.[29][30] Some non-desert cicada species such as Magicicada tredecem also cool themselves evaporatively, but less dramatically.[31] Conversely, many other cicadas can voluntarily raise their body temperatures as much as 22 °C (40 °F) above ambient temperature.[32]
Song
The "singing" of male cicadas is produced principally and in the majority of species using a special structure called a tymbal, a pair of which lies below each side of the anterior abdominal region. The structure is buckled by muscular action and being made of resilin unbuckled rapidly on muscle relaxation and the rapid action of muscles produces their characteristic sounds. Some cicadas, however, have mechanisms for stridulation, sometimes in addition to the tymbals. Here, the wings are rubbed over a series of midthoracic ridges. The sounds may further be modulated by membranous coverings and by resonant cavities.[23] The male abdomen in some species is largely hollow, and acts as a sound box. By rapidly vibrating these membranes, a cicada combines the clicks into apparently continuous notes, and enlarged chambers derived from the tracheae serve as resonance chambers with which it amplifies the sound. The cicada also modulates the song by positioning its abdomen toward or away from the substrate. Partly by the pattern in which it combines the clicks, each species produces its own distinctive mating songs and acoustic signals, ensuring that the song attracts only appropriate mates.[14]
Average temperature of the natural habitat for the South American species Fidicina rana is about 29 °C (84 °F). During sound production, the temperature of the tymbal muscles was found to be significantly higher.[33] Many cicadas sing most actively during the hottest hours of a summer day; roughly a 24-hour cycle.[34] Most cicadas are diurnal in their calling and depend on external heat to warm them up, while a few are capable of raising their temperatures using muscle action and some species are known to call at dusk.[35] Kanakia gigas and Froggattoides typicus are among the few that are known to be truly nocturnal and there may be other nocturnal species living in tropical forests.[36][37]
Cicadas call from varying heights on trees. Where multiple species occur, the species may use different heights and timing of calling.[38][39] While the vast majority of cicadas call from above the ground, two Californian species, Okanagana pallidula and O. vanduzeei are known to call from hollows made at the base of the tree below the ground level. The adaptive significance is unclear as the calls are not amplified or modified by the burrow structure but it is thought that this may avoid predation.[40]
Although only males produce the cicadas' distinctive sounds, both sexes have membranous structures called tympana by which they detect sounds, the equivalent of having ears. Males disable their own tympana while calling, thereby preventing damage to their hearing;[41] a necessity partly because some cicadas produce sounds up to 120 dB (SPL)[41] which is among the loudest of all insect-produced sounds.[42] The song is loud enough to cause permanent hearing loss in humans should the cicada be at "close range". In contrast, some small species have songs so high in pitch that they are inaudible to humans.[43]
For the human ear, telling precisely where a cicada song originates is often difficult. The pitch is nearly constant, the sound is continuous to the human ear, and cicadas sing in scattered groups. In addition to the mating song, many species have a distinct distress call, usually a broken and erratic sound emitted by the insect when seized or panicked. Some species also have courtship songs, generally quieter, and produced after a female has been drawn to the calling song. Males also produce encounter calls, whether in courtship or to maintain personal space within choruses.[44]
The song of cicadas is considered by entomologists to be unique to a given species, and a number of resources exist to collect and analyse cicada sounds.[45]
Lifecycle
In some species of cicada, the males remain in one location and call to attract females. Sometimes several males aggregate and call in chorus. In other species, the males move from place to place, usually with quieter calls while searching for females. The Tettigarctidae differ from other cicadas in producing vibrations in the substrate rather than audible sounds.[9] After mating, the female cuts slits into the bark of a twig where she deposits her eggs.[9]
When the eggs hatch, the newly hatched nymphs drop to the ground and burrow. Cicadas live underground as nymphs for most of their lives at depths down to about 2.5 m (8 ft). Nymphs have strong front legs for digging and excavating chambers in close proximity to roots where they feed on xylem sap. In the process, their bodies and interior of the burrow become coated in anal fluids. In wet habitats, larger species construct mud towers above ground to aerate their burrows. In the final nymphal instar, they construct an exit tunnel to the surface and emerge.[9] They then moult (shed their skins) on a nearby plant for the last time, and emerge as adults. The exuviae or abandoned exoskeletons remain, still clinging to the bark of the tree.[46]
Most cicadas go through a lifecycle that lasts from two to five years. Some species have much longer lifecycles, such as the North American genus, Magicicada, which has a number of distinct "broods" that go through either a 17-year, or in some parts of the region, a 13-year lifecycle. The long lifecycles may have developed as a response to predators, such as the cicada killer wasp and praying mantis.[47][48][49] A specialist predator with a shorter life cycle of at least two years could not reliably prey upon the cicadas.[50] An alternate hypothesis is that these long lifecycles evolved during the ice ages so as to overcome cold spells and that as species co-emerged and hybridized they left distinct species that did not hybridize having periods matching prime numbers.[51]
 An adult cicada that has just emerged and is waiting to dry before flying away
An adult cicada that has just emerged and is waiting to dry before flying away Newly emerged adult cicada held on human fingers
Newly emerged adult cicada held on human fingers Cicada exuviae after the adult cicada has left
Cicada exuviae after the adult cicada has left
Diet
Cicada nymphs drink sap from the xylem of various species of trees, including oak, cypress, willow, ash, and maple. While common folklore indicates that adults do not eat, they actually do drink plant sap using their sucking mouthparts.[52][53]
Locomotion
Cicadas, unlike other Auchenorrhyncha, are not adapted for jumping (saltation).[54] They have the usual insect modes of locomotion, walking and flight. However, they do not walk or run well, and take to the wing to travel distances greater than a few centimetres.[9]
Predators, parasites and pathogens
_with_Cicada.jpg)
Cicadas are commonly eaten by birds and sometimes by squirrels,[55] as well as bats, wasps, mantises, spiders, and robber flies. In times of mass emergence of cicadas, various amphibians, fish, reptiles, mammals, and birds change their foraging habits so as to benefit from the glut. Newly hatched nymphs may be eaten by ants, and nymphs living underground are preyed on by burrowing mammals such as moles.[25] In Australia, cicadas are preyed on by the Australian cicada killer wasp (Exeirus lateritius), which stings and stuns cicadas high in the trees, making them drop to the ground, where the cicada-hunter mounts and carries them, pushing with its hind legs, sometimes over a distance of a 100 m, until they can be shoved down into its burrow, where the numb cicadas are placed onto one of many shelves in a "catacomb", to form the food-stock for the wasp grub that grows out of the egg deposited there.[56] A katydid predator from Australia is capable of attracting singing male cicadas of a variety of species by imitating the timed click replies of sexually receptive female cicadas, which respond in pair-formation by flicking their wings.[57]
Several fungal diseases infect and kill adult cicadas, while other entomopathogenic fungus, Ophiocordyceps and Isaria spp., attacks nymphs.[25] Massospora cicadina specifically attacks the adults of periodical cicadas, the spores remaining dormant in the soil between outbreaks.[58] This fungus is also capable of dosing cicadas with psilocibin, the psychedelic drug found in magic mushrooms, as well as cathinone, an alkaloid similar to various amphetamines. These chemicals alter the behaviour of the cicadas, driving males to copulate, even with males, and is thought to be beneficial to the fungus as the fungal spores are dispersed by a larger number of infected carriers.[59]
Antipredator adaptations

Cicadas use a variety of strategies to evade predators. Large cicadas can fly rapidly to escape if disturbed.[60] Many are extremely well camouflaged[60][61] to evade predators such as birds that hunt by sight. As well as being coloured like tree bark, they are disruptively patterned to break up their outlines;[62] their partly transparent wings are held over the body and pressed close to the substrate. Some cicada species play dead when threatened.[63][64]
The periodical cicadas (Magicicada) make use of predator satiation: they emerge, all at once, at long intervals of 13 or 17 years; their juveniles are probably the longest-lived of all insect development stages.[65] Since the cicadas in any given area exceeds the number predators can eat, all available predators are sated, and the remaining cicadas can breed in peace.[60][65]
Some cicadas such as Hemisciera maculipennis display bright deimatic flash coloration on their hind wings when threatened; the sudden contrast helps to startle predators, giving the cicadas time to escape.[66] The majority of cicadas are diurnal and rely on camouflage when at rest, but some species use aposematism-related Batesian mimicry, wearing the bright colors that warn of toxicity in other animals; the Malaysian Huechys sanguinea has conspicuous red and black warning coloration, is diurnal, and boldly flies about in full view of possible predators.[67]

Predators such as the sarcophagid fly Emblemasoma hunt cicadas by sound, being attracted to their songs.[68] Singing males soften their song so that the attention of the listener gets distracted to neighbouring louder singers, or cease singing altogether as a predator approaches. A loud cicada song, especially in chorus, has been asserted to repel predators, but observations of predator responses refute the claim.[65]
In human culture
In art and literature
.jpg)

Cicadas have been featured in literature since the time of Homer's Iliad, and as motifs in decorative art from the Chinese Shang dynasty (1766–1122 BC.).[lower-alpha 4] They are described by Aristotle in his History of Animals and by Pliny the Elder in his Natural History; their mechanism of sound production is mentioned by Hesiod in his poem "Works and Days": "when the Skolymus flowers, and the tuneful Tettix sitting on his tree in the weary summer season pours forth from under his wings his shrill song".[70] In the classic 14th-century Chinese novel Romance of the Three Kingdoms, Diaochan took her name from the sable (diāo) tails and jade decorations in the shape of cicadas (chán), which adorned the hats of high-level officials. In the Japanese novel The Tale of Genji, the title character poetically likens one of his many love interests to a cicada for the way she delicately sheds her robe the way a cicada sheds its shell when molting. A cicada exuvia plays a role in the manga Winter Cicada. Cicadas are a frequent subject of haiku, where, depending on type, they can indicate spring, summer, or autumn.[71] Shaun Tan's illustrated book Cicada tells the story of a hardworking but underappreciated cicada working in an office.[72]
In music
Cicadas are featured in the well-known protest song "Como La Cigarra" ("Like the Cicada") written by Argentinian poet and composer María Elena Walsh. In the song, the cicada is a symbol of survival and defiance against death. The song was famously recorded by Mercedes Sosa, among other Latin American musicians. Another well-known song, "La Cigarra" ("The Cicada"), written by Raymundo Perez Soto, is a song in the mariachi tradition that romanticises the insect as a creature that sings until it dies.[73]
The Brazilian artist Lenine with his track "Malvadeza" from the album "Chão" creates a song built upon the sound of the cicada that can be heard along the track.[74]
In mythology and folklore
Cicadas have been used as money, in folk medicine, to forecast the weather, to provide song (in China), and in folklore and myths around the world.[75] In France, the cicada represents the folklore of Provence and the Mediterranean cities.[76]
The cicada has represented insouciance since classical antiquity. Jean de La Fontaine began his collection of fables Les fables de La Fontaine with the story "La Cigale et la Fourmi" ("The Cicada and the Ant") based on one of Aesop's fables; in it, the cicada spends the summer singing, while the ant stores away food, and finds herself without food when the weather turns bitter.[77]
The cicada symbolises rebirth and immortality in Chinese tradition.[78] In the Chinese essay "Thirty-Six Stratagems", the phrase "to shed the golden cicada skin" (simplified Chinese: 金蝉脱壳; traditional Chinese: 金蟬脫殼; pinyin: jīnchán tuōqiào) is the poetic name for using a decoy (leaving the exuvia) to fool enemies.[79] In the Chinese classic novel Journey to the West (16th century), the protagonist Priest of Tang was named the Golden Cicada.[80]
In Japan, the cicada is associated with the summer season.[81] For many Japanese people, summer hasn't officially begun until the first songs of the cicada are heard.[82] According to Lafcadio Hearn, the song of Meimuna opalifera, called tsuku-tsuku boshi, is said to indicate the end of summer, and it is called so because of its particular call.[83]
In the Homeric Hymn to Aphrodite, the goddess Aphrodite retells the legend of how Eos, the goddess of the dawn, requested Zeus to let her lover Tithonus live forever as an immortal.[84] Zeus granted her request, but because Eos forgot to ask him to also make Tithonus ageless, Tithonus never died, but he did grow old.[84] Eventually, he became so tiny and shriveled that he turned into the first cicada.[84] The Greeks also used a cicada sitting on a harp as an emblem of music.[85]
In Kapampangan mythology in the Philippines, the goddess of dusk, Sisilim, is said to be greeted by the sounds and appearances of cicadas whenever she appears.[86]

As food and folk medicine
Cicadas were eaten in Ancient Greece, and are consumed today in China, both as adults and (more often) as nymphs.[87] Cicadas are also eaten in Malaysia, Burma, North America, and central Africa, as well as the Balochistan region of Pakistan, especially in Ziarat.[88] Female cicadas are prized for being meatier.[43] Shells of cicadas are employed in traditional Chinese medicines.[89] The 17-year "Onondaga Brood"[90] Magicicada is culturally important and a particular delicacy to the Onondaga people.[91]
As pests
Cicadas feed on sap; they do not bite or sting in a true sense, but may occasionally mistake a person's arm for a plant limb and attempt to feed.[92] Male cicadas produce very loud calls that can damage human hearing.[93]
Cicadas are not major agricultural pests, but in some outbreak years, trees may be overwhelmed by the sheer numbers of females laying their eggs in the shoots. Small trees may wilt and larger trees may lose small branches.[25] Although in general, the feeding activities of the nymphs do little damage, during the year before an outbreak of periodic cicadas, the large nymphs feed heavily and plant growth may suffer.[94] Some species have turned from wild grasses to sugarcane, which affects the crop adversely, and in a few isolated cases, females have oviposited on food crops such as date palms, grape vines, citrus trees, asparagus, and cotton.[25]
Cicadas sometimes cause damage to amenity shrubs and trees, mainly in the form of scarring left on tree branches where the females have laid their eggs. Branches of young trees may die as a result.[95][96]
See also
Notes
References
- Fitzgerals, Kevin (22 March 2016). "How Do Cicadas Know When to Emerge from the Ground?". Entomology Today. Retrieved 24 July 2017.
- "cicada". Merriam-Webster. Retrieved 16 January 2018.
- "Words To Remember Every 13 Years Or So". Dictionary.com. Retrieved 16 January 2018.
- Moulds, MS (2005). "An appraisal of the higher classification of cicadas (Hemiptera: Cicadoidea) with special reference to the Australian fauna" (PDF). Records of the Australian Museum. 57 (3): 375–446. doi:10.3853/j.0067-1975.57.2005.1447.
- Marshall, DC; Moulds, M; Hill, KBR; Price, BW; Wade, EJ; Owen, CO; Goemans, G; Marathe, K; Sarkar, V; Cooley, JR; Sanborn, AF; Kunte, K; Villet, MH; Simon, C (2018). "A molecular phylogeny of the cicadas (Hemiptera: Cicadidae) with a review of tribe and subfamily classification". Zootaxa. 4424 (1): 1–64. doi:10.11646/zootaxa.4424.1.1. PMID 30313477.
- Carwardine, Mark (2008). Animal Records. Sterling Publishing. p. 224. ISBN 978-1-4027-5623-8.
- Burton, Maurice; Burton, Robert (2002). International Wildlife Encyclopedia: Chickaree - crabs. Marshall Cavendish. pp. 455–457. ISBN 978-0-7614-7270-4.
- Snodgrass, Robert Evans. Insects Their Ways and Means of Living (1st ed.). Smithsonian. p. Facing page 198.
- Resh, Vincent H.; Cardé, Ring T. (2009). Encyclopedia of Insects. Academic Press. pp. 56–63. ISBN 978-0-08-092090-0.
- Sanborn, Allen F.; Phillips, Polly K. (2013). "Biogeography of the Cicadas (Hemiptera: Cicadidae) of North America, North of Mexico". Diversity. 5 (2): 166–239. doi:10.3390/d5020166.
- Hogue, Charles Leonard (1993). Latin American Insects and Entomology. University of California Press. p. 233. ISBN 978-0-520-07849-9.
- Simon, Chris (2015). "The Current Status of Cicada Taxonomy on a Region-by-Region Basis". University of Connecticut. Retrieved 27 August 2015. Cite journal requires
|journal=(help) - Marren, Peter; Mabey, Richard (2010). Bugs Britannica. Chatto and Windus. p. 189. ISBN 978-0-7011-8180-2.
- Milne, Lorus; Milne, Margery (1992). The Audubon Society Field Guide to North American Insects and Spiders. Alfred A Knopf. ISBN 978-0-394-50763-7.
- "Periodical Cicadas". magicicada.org.
- "Cicadas: Superfamily Cicadoidea". Australian Museum. Retrieved 30 July 2016.
- 1. Introducing cicadas – Cicadas – Te Ara Encyclopedia of New Zealand
- Wang, Bo; Zhang, Haichun; Szwedo, Jacek (2009). "Jurassic Palaeontinidae From China and the Higher Systematics of Palaeontinoidea (Insecta: Hemiptera: Cicadomorpha)". Palaeontology. 52 (1): 53–64. doi:10.1111/j.1475-4983.2008.00826.x.
- Szwedo, Jacek (January 2018). "The unity, diversity and conformity of bugs (Hemiptera) through time". Earth and Environmental Science Transactions of the Royal Society of Edinburgh. 107 (2–3): 109–128. doi:10.1017/s175569101700038x. ISSN 1755-6910.
- Arthur Gardiner Butler (1869–1874). Lepidoptera Exotica; or, Descriptions and Illustrations of Exotic Lepidoptera. E. W. Jansen. pp. 126–127.
- Moulds, M.S. 2018. Cicada fossils (Cicadoidea: Tettigarctidae and Cicadidae) with a review of the named fossilised Cicadidae. Zootaxa 4438(3): 443–470. https://doi.org/10.11646/zootaxa.4438.3.2
- Poinar, G. & Kritsky, G. (2011) Morphological conservatism in the foreleg structure of cicada hatchlings, Burmacicada protera n. gen., n. sp. in Burmese amber, Dominicicada youngi n. gen., n. sp. in Dominican amber and the extant Magicicada septendecim (L.) (Hemiptera: Cicadidae). Historical Biology, 24, 461–466. https://doi.org/10.1080/08912963.2011.603421
- Cuvier, Georges; Blyth, Edward; Mudie, Robert; Johnston, George; Westwood, John Obadiah; Carpenter, William Benjamin (1851). The Animal Kingdom: Arranged After Its Organization, Forming a Natural History of Animals, and an Introduction to Comparative Anatomy. W. S. Orr and Company. pp. 567–570.
- Flindt, R. (2006). Amazing Numbers in Biology, p. 10. ISBN 978-3-540-30146-2
- Capinera, John L. (2008). Encyclopedia of Entomology. Springer Science & Business Media. p. 876. ISBN 978-1-4020-6242-1.
- Moulds, Maxwell Sydney (1990). Australian Cicadas. New South Wales University Press. p. 10. ISBN 978-0-86840-139-3.
- Choi, Charles Q. (29 April 2013). "Cicada Wings Are Self-Cleaning". Scientific American. Retrieved 3 September 2015.
- Yirka, Bob (6 March 2013). "Researchers find cicada wing structure able to kill bacteria on contact". Phys.org. Retrieved 3 September 2015.
- Hadley, Neil F.; Quinlan, Michael C.; Kennedy, Michael L. (1991). "Evaporative cooling in the desert cicada: thermal efficiency and water/metabolic costs". Journal of Experimental Biology. 159 (1): 269–283.
- Toolson, Eric C. (1987). "Water Profligacy as an Adaptation to Hot Deserts: Water Loss Rates and Evaporative Cooling in the Sonoran Desert Cicada, Diceroprocta apache". Physiological Zoology. 60 (4): 379–385. doi:10.1086/physzool.60.4.30157899.
- Toolson, Eric C.; Toolson, Elizabeth K. (1991). "Evaporative cooling and endothermy in the 13-year periodical cicada, Magicicada tredecem". Journal of Comparative Physiology B. 161 (1): 109–115. doi:10.1007/BF00258754.
- Sanborn, Allen F.; Villet, Martin H.; Phillips, Polly K. (2003). "Hot-blooded singers: endothermy facilitates crepuscular signaling in African platypleurine cicadas (Homóptera: Cicadidae: Platypleura spp.)". Naturwissenschaften. 90 (7): 305–308. Bibcode:2003NW.....90..305S. doi:10.1007/s00114-003-0428-1. PMID 12883772.
- Aidley, D. J.; White, D.C.S. (1969). "Mechanical properties of glycerinated fibres from the tymbal muscles of a Brazilian cicada". Journal of Physiology. 205 (1): 179–192. doi:10.1113/jphysiol.1969.sp008959. PMC 1348633. PMID 5347716.
- Turpin, Tom (11 September 2008). "Sound of Insect Music Ushers in Fall Season". Purdue University. Archived from the original on 20 September 2015. Retrieved 27 August 2015.
- Sanborn, Allen F; Villet, Martin H; Phillips, Polly K (2003). "Hot-blooded singers: Endothermy facilitates crepuscular signaling in African platypleurine cicadas (Hemiptera: Cicadidae: Platypleura spp.)". Naturwissenschaften. 90 (7): 305–308. Bibcode:2003NW.....90..305S. doi:10.1007/s00114-003-0428-1. PMID 12883772. S2CID 9090814.
- Drosopoulos, Sakis; Claridge, Michael F. (2005). Insect Sounds and Communication: Physiology, Behaviour, Ecology, and Evolution. CRC Press. p. 348.
- Ewart, A.; Popple, L.W. (2007). "Songs and calling behaviour of Froggattoides typicus Distant (Hemiptera: Cicadoidea: Cicadidae), a nocturnally singing cicada". Australian Entomologist. 34 (4): 127–139.
- Sueur, J.; Aubin, T. (1 July 2003). "Is microhabitat segregation between two cicada species ( Tibicina haematodes and Cicada orni ) due to calling song propagation constraints?". Naturwissenschaften. 90 (7): 322–326. Bibcode:2003NW.....90..322S. doi:10.1007/s00114-003-0432-5. ISSN 0028-1042. PMID 12883776. S2CID 25048233.
- Sueur, Jerome (2002). "Cicada acoustic communication: potential sound partitioning in a multispecies community from Mexico (Hemiptera: Cicadomorpha: Cicadidae)". Biological Journal of the Linnean Society. 75 (3): 379–394. doi:10.1046/j.1095-8312.2002.00030.x. ISSN 0024-4066.
- Sanborn, Allen F.; Phillips, Polly K. (1995). "No acoustic benefit to subterranean calling in the cicada Okanagana pallidula Davis(Homoptera: Tibicinidae)". Great Basin Naturalist. 55 (4): 374–376.
- "Cicada noise". 50/50. NZ. 2 June 2002. Archived from the original on 4 October 2006. Retrieved 3 August 2011.
- Craig, Owen (17 February 2001). "Summer of singing cicadas". Australia: ABC Science. Retrieved 23 December 2006.
- "Arthropoda". Insect education. 9 September 2008. Archived from the original on 13 July 2011. Retrieved 20 September 2009.
- Villet, Martin H.; Sanborn, Allen F.; Phillips, Polly K. (2003). "Endothermy and chorusing behaviour in the African platypleurine cicada Pycna semiclara (Germar, 1834) (Hemiptera: Cicadidae)". Canadian Journal of Zoology. 81 (8): 1437–1444. doi:10.1139/z03-119.
- Baker, E.; Price, B. W.; Rycroft, S. D.; Villet, M. H. (2015). "Global Cicada Sound Collection I: Recordings from South Africa and Malawi by B. W. Price & M. H. Villet and harvesting of BioAcoustica data by GBIF". Biodiversity Data Journal. 3 (3): e5792. doi:10.3897/BDJ.3.e5792. PMC 4568402. PMID 26379465.
- Grimaldi, David; Engel, Michael S. (2005). Evolution of the Insects. Cambridge University Press. p. 308. ISBN 978-1-107-26877-7.
- Haga, Enoch (1994–2007). "6. Eratosthenes goes bugs!". Exploring Prime Numbers on Your PC and the Internet. Enoch Haga. pp. 71–80, fig. 8, table 9. ISBN 978-1-885794-24-6. LCCN 2007900755..
- Haga, Enoch (2009). "Sequence A161664, Safe periods for the emergence of cicada species on prime number cycles". In Sloane, NJA (ed.). The On-Line Encyclopedia of Integer Sequences..
- "Scanjet image of 7-petal flower from Jade plant approximately 25 years or more years old in Livermore, California" (JPEG) (image). Wikimedia. Retrieved 20 September 2009.
- Dawkins, Richard (1986). The Blind Watchmaker. W. W. Norton & Company. p. 100. ISBN 978-0-393-31570-7.
- Yoshimura, J. (1997). "The Evolutionary Origins of Periodical Cicadas During Ice Ages". The American Naturalist. 149 (1): 112–124. doi:10.1086/285981.
- Elliott, Lang, and Wil Hershberger. 2007. The Songs of Insects. Boston: Houghton Mifflin Co. pp. 184–185. ISBN 0618663975.
- Periodical Cicadas – Genus Magicicada
- Burrows, M. (March 2013). "Jumping mechanisms of treehopper insects (Hemiptera, Auchenorrhyncha, Membracidae)". Journal of Experimental Biology. 216 (5): 788–799. doi:10.1242/jeb.078741. PMID 23155084.
- Marlatt, C. L. (1898). The Periodical Cicada. Washington: U.S. Government Printing Office. p. 106.
- Tillyard, R. J. (1926). The Insects of Australia and New Zealand. Sydney: Angus & Robertson. pp. 298–299.
- Marshall, D.C. & Hill, K.B.R. 2009. Versatile aggressive mimicry of cicadas by an Australian predatory katydid. PLoS One 4(1): e4185. doi:10.1371/journal.pone.0004185
- Speare, A. T. (1921). "Massospora cicadina Peck: A Fungous Parasite of the Periodical Cicada". Mycologia. 13 (2): 72–82. doi:10.2307/3753297. JSTOR 3753297.
- Boyce, Greg R.; Gluck-Thaler, Emile; Slot, Jason C.; Stajich, Jason E.; Davis, William J.; James, Tim Y.; Cooley, John R.; Panaccione, Daniel G.; Eilenberg, Jørgen (2019). "Psychoactive plant- and mushroom-associated alkaloids from two behavior modifying cicada pathogens". Fungal Ecology. 41: 147–164. doi:10.1016/j.funeco.2019.06.002. PMC 6876628. PMID 31768192.
- Brown, David (4 May 2004). "Cicadas' bizarre survival strategy". NBC News. The Washington Post. Retrieved 24 August 2015.
- "Animals disappear using camouflage". The Telegraph. Retrieved 24 August 2015.
- Goldman, Jason G. "1-Trick Chameleon: Predators Learn to See Through Camouflage". Nautilus. Retrieved 24 August 2015.
- "Adult Cicada Defenses". Massachusetts Cicadas. Retrieved 5 September 2017.
- "Subspecies Neotibicen lyricen lyricen - Lyric Cicada ("ssp. lyricen var. lyricen")". BugGuide.Net. Retrieved 5 September 2017.
- Williams, Kathy S.; Simon, Chris (1995). "The Ecology, Behavior, And Evolution of Periodical Cicadas" (PDF). Annual Review of Entomology. 40: 269–295. doi:10.1146/annurev.ento.40.1.269.
- Cott, Hugh B. (1940). Adaptive Coloration in Animals. Methuen. pp. 375–376.
- Cott, Hugh B. (1940). Adaptive Coloration in Animals. Methuen. p. 203.
- Zuk, Marlene; Kolluru, Gita R. (December 1998). "Exploitation of Sexual Signals by Predators and Parasitoids". Quarterly Review of Biology. 73 (4): 415–438. doi:10.1086/420412.
- "Cicada". Asian Art Museum.
- Myers, J. G. (1929). Insect Singers (PDF). G. Routledge and Sons.
- "Cicadas". Haiku topical dictionary. University of Virginia. Archived from the original on 12 December 2012..
- "Cicada". Arthur A. Levine Books. Retrieved 20 February 2019.
- "La Cigarra / The Cicada". Lyrics Translate. Retrieved 24 August 2015.
- "Malvadeza". Letras. Retrieved 4 November 2019.
- "Cicada". Britannica. Retrieved 12 July 2015.
- "La cigale, emblème de la Provence" (in French). FR: Notre Provence. Archived from the original on 15 March 2009.
- Chevrier, Irène (24 April 2007). "La Fontaine, fabuleusement inspiré par Esope – Un autre regard sur la Grèce" (in French). Archived from the original on 28 December 2008.
- Riegel, Garland. "Cicada in Chinese Folklore (with bibliography)". Insects.org. Archived from the original on 5 September 2015. Retrieved 24 August 2015.
- "Thirty-Six Strategies". Wengu. Retrieved 31 January 2016.
- Yu, Anthony C. Yu (trans.), ed. (1977). The Journey to the West. I. University of Chicago Press. p. 14.
- "The Cicadas' Song: Japan's Summer Soundtrack". Retrieved 24 August 2015.
- "Cicadas". Japan Experience. 31 May 2017. Retrieved 11 November 2018.
- Hearn, Lafcadio (2007). Shadowings. Cosimo. p. 85. ISBN 978-1-60206-066-1.
- DuBois, Page (2010). Out of Athens: The New Ancient Greeks. Cambridge, Massachusetts: Harvard University Press. pp. 51–53. ISBN 978-0-674-03558-4.CS1 maint: ref=harv (link)
- "The Cicada". The Sydney Morning Herald. National Library of Australia. 21 January 1928. p. 21. Retrieved 7 June 2013.
- "Formation of the World, Kapampangan Mythology". Aswang Project.
- Simoons, Frederick J. (1990). Food in China: A Cultural and Historical Inquiry. CRC Press. p. 334. ISBN 978-0-8493-8804-0.
- "Taazan – Cicada – How to Cook ? – Ziarat", YouTube.com (in Urdu), Balochistan, Pakistan, retrieved 25 August 2019
- Li Shizhen, Bencao Gangmu, Section of Insect. 李时珍, 本草纲目, 虫部
- "Brood VII: The Onondaga Brood". Periodical Cicadas. Retrieved 1 June 2018.
- Dehowähda•dih. "Ogweñ•yó'da' déñ'se' Hanadagá•yas: The Cicada and George Washington". Onondaga Nation Website. Retrieved 1 June 2018.
- "Do cicadas bite or sting?". Cicada Mania. 28 June 2008. Archived from the original on 11 June 2018. Retrieved 15 July 2017..
- "Cicadas | National Geographic". Retrieved 15 July 2017.
- Yang, Louie H. (2004). "Periodical cicadas as resource pulses in North American forests". Science. 306 (5701): 1565–1567. Bibcode:2004Sci...306.1565Y. doi:10.1126/science.1103114. PMID 15567865.
- "Great Damage from Insects; They Are Doing More Destruction to Crops This Year than Usual". The New York Times. 9 September 1895. p. 6..
- "Ohio Cultivator". 3 (1). Columbus, Ohio. 1 January 1847: 3–. Retrieved 30 March 2013. Cite journal requires
|journal=(help)
Further reading
- Clausen, Lucy W. (1954). Insect Fact and Folklore. Macmillan.
- Egan, Rory B. (1994). "Cicada in Ancient Greece". Archived from the original on 10 November 2006. Retrieved 28 December 2006.
- Hoppensteadt, Frank C; Keller, Joseph B (1976). "Synchronization of periodical cicada emergences" (PDF). Science. 194 (4262): 335–337. Bibcode:1976Sci...194..335H. doi:10.1126/science.987617. PMID 987617.
- Myers, JG (1929). Insect Singers: A Natural History of the Cicadas. Routledge.
- Ramel, Gordon (2005). "The Singing Cicadas". Earth life. Retrieved 31 January 2007.
- Riegel, Garland (November 1994). Cicada in Chinese Folklore. Melsheimer Entomological Series. Bug bios. Archived from the original on 10 November 2006. Retrieved 28 December 2006.
- Walker, Annette (2000). The Reed Handbook of Common New Zealand Insects. Reed. ISBN 978-0-7900-0718-2.
External links
| Wikimedia Commons has media related to Cicadoidea. |
| Wikispecies has information related to cicadidae |
| Look up cicada in Wiktionary, the free dictionary. |
- Cicada Mania – Website dedicated to cicadas, the most amazing insects in the world
- Massachusetts Cicadas describes behavior, sightings, photos, how to find guide, videos, periodical and annual cicada species information and distribution maps
- Magicicada.org Brood mapping project – solicits records and observations from the general public
- Song recordings and information of cicadas of the United States and Canada
- Cicadas of Florida, Neocicada hieroglyphica, Tibicen, Diceroprocta and Melampsalta spp. at University of Florida/IFAS Featured Creatures
- Greater Cincinnati Cicada Information & Teaching Resources – College of Mt Saint Joseph Cicada Information Site
- DrMetcalf: a resource on cicadas, leafhoppers, planthoppers, spittlebugs, and treehoppers