Mealybug
Mealybugs are insects in the family Pseudococcidae, unarmored scale insects found in moist, warm habitats. Many species are considered pests by some humans as they feed on plant juices of greenhouse plants, house plants and subtropical trees and also act as a vector for several plant diseases. Some ants, however live in symbiotic relationships with them.
| Pseudococcidae | |
|---|---|
 | |
| Mealybugs on a flower stem in Yogyakarta | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Hemiptera |
| Suborder: | Sternorrhyncha |
| Superfamily: | Coccoidea |
| Family: | Pseudococcidae Heymons, 1915 [1] |
Description
Mealybugs are sexually dimorphic: females appear as nymphs, exhibiting reduced morphology, and lack wings, although unlike many female scale insects, they often retain legs and can move. Males are smaller, gnat-like and have wings. Since mealybugs (as well as all other Hemiptera) are hemimetabolous insects, they do not undergo complete metamorphosis in the true sense of the word. However, male mealybugs do exhibit a radical change during their life cycle, changing from wingless, ovoid nymphs to wasp-like flying adults.
Mealybug females feed on plant sap, normally in roots or other crevices, and in a few cases the bottoms of stored fruit. They attach themselves to the plant and secrete a powdery wax layer (hence the name "mealy" bug) used for protection while they suck the plant juices. In Asia, mango mealybug is considered a major menace for the mango crop. The males on the other hand are short-lived as they do not feed at all as adults and only live to fertilize the females. Male citrus mealy bugs fly to the females and resemble fluffy gnats.
Some species of mealybug lay their eggs in the same waxy layer used for protection in quantities of 50–100; other species are born directly from the female.
The most serious pests are mealybugs that feed on citrus; other species damage sugarcane, grapes, pineapple (Jahn et al. 2003), coffee trees, cassava, ferns, cacti, gardenias, papaya, mulberry, sunflower and orchids. Mealybugs only tend to be serious pests in the presence of ants because the ants protect them from predators and parasites.[2] Mealybugs also infest some species of carnivorous plant such as Sarracenia (pitcher plants); in such cases it is difficult to eradicate them without repeated applications of insecticide such as diazinon. Small infestations may not inflict significant damage. In larger amounts though, they can induce leaf drop. In recent years, some of the mealybug species have become invasive pests in localities posing a great problem to the new agro-ecosystems. In India, Withania somnifera plant have been reported as a new reservoir host for an invasive mealybug species Phenacoccus solenopsis.[3]
Fossil specimens of genus Acropyga ants have been recovered from the Burdigalian stage Dominican amber deposits and several individuals are preserved carrying the extinct mealybug genus Electromyrmococcus.[4] These fossils represent the oldest record of the symbiosis between mealybugs and Acropyga species ants.[4]
 Male hibiscus mealybug, Maconellicoccus hirsutus
Male hibiscus mealybug, Maconellicoccus hirsutus Formica fusca ants tending a herd of mealybugs
Formica fusca ants tending a herd of mealybugs.jpg) A ladybird preying on mealybugs
A ladybird preying on mealybugs Mealybugs on hibiscus plant
Mealybugs on hibiscus plant Phenacoccus aceris
Phenacoccus aceris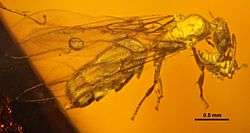 Acropyga glaesaria ant carrying an Electromyrmococcus abductus mealybug
Acropyga glaesaria ant carrying an Electromyrmococcus abductus mealybug
Control methods
- If the infested plant can tolerate the cold, the plant can be placed on a windowsill during cold weather. This will cause the mealybugs to move to the leaf furthest from the window, where they can be wiped off with a cloth.
- Ladybird larvae and adults feed on mealybugs, and can be used to control an infestation.
- Mealybugs can be controlled using the fungus Lecanicillium lecanii.
- Insecticidal soap is effective against mealybugs. It is commercially available and can be purchased online or at a supermarket.
- An aqueous solution containing 50% v/v isopropyl alcohol and 1% w/v sodium dodecyl sulfate (found in most household detergents) is effective against mealybugs. Isopropyl alcohol dissolves the outer waxy covering, while the sodium dodecyl sulfate attacks the mealybugs themselves.
- Diatomaceous earth can be applied around the stem of the plant. Diatomaceous earth contains small silica particles that are trapped within the joints of ants. They cause irritation and eventual death. Diatomaceous earth is especially useful for an infestation that has developed a symbiotic relationship with local ants, as is often the case.
- Diazinon can be used, but often requires multiple applications.
Genera
- Acaciacoccus
- Acinicoccus
- Acrochordonus
- Adelosoma
- Agastococcus
- Albertinia
- Allomyrmococcus
- Allotrionymus
- Amonostherium
- Anaparaputo
- Anisococcus
- Annulococcus
- Anthelococcus
- Antonina
- Antoninella
- Antoninoides
- Apodastococcus
- Artemicoccus
- Asaphococcus
- Asphodelococcus
- Asteliacoccus
- Atriplicicoccus
- Atrococcus
- Australicoccus
- Australiputo
- Balanococcus
- Bessenayla
- Bimillenia
- Birendracoccus
- Boninococcus
- Boreococcus
- Bouhelia
- Brasiliputo
- Brevennia
- Brevicoccus
- Callitricoccus
- Calyptococcus
- Cannococcus
- Capitisetella
- Cataenococcus
- Chaetococcus
- Chaetotrionymus
- Chileputo
- Chlorococcus
- Chnaurococcus
- Chorizococcus
- Chryseococcus
- Cintococcus
- Circaputo
- Cirnecoccus
- Clavicoccus
- Coccidohystrix
- Coccura
- Coleococcus
- Colombiacoccus
- Conicosoma
- Conulicoccus
- Coorongia
- Cormiococcus
- Criniticoccus
- Crisicoccus
- Crocydococcus
- Cryptoripersia
- Cucullococcus
- Cyperia
- Cypericoccus
- Cyphonococcus
- Dawa
- Delococcus
- Delottococcus
- Densispina
- Discococcus
- Distichlicoccus
- Diversicrus
- Drymococcus
- Dysmicoccus
- Eastia
- Ehrhornia
- Electromyrmococcus †
- Epicoccus
- Erimococcus
- Eriocorys
- Erioides
- Erium
- Eucalyptococcus
- Eumirococcus
- Eumyrmococcus
- Eupeliococcus
- Euripersia
- Eurycoccus
- Exilipedronia
- Farinococcus
- Ferrisia
- Ferrisicoccus
- Fijicoccus
- Fonscolombia
- Formicococcus
- Gallulacoccus
- Geococcus
- Glycycnyza
- Gomezmenoricoccus
- Gouxia
- Greenoripersia
- Grewiacoccus
- Hadrococcus
- Heliococcus
- Heterococcopsis
- Heterococcus
- Heteroheliococcus
- Hippeococcus
- Hopefoldia
- Humococcus
- Hypogeococcus
- Iberococcus
- Idiococcus
- Indococcus
- Inopicoccus
- Ityococcus
- Kenmorea
- Kermicus
- Kiritshenkella
- Lachnodiella
- Lachnodiopsis
- Lacombia
- Laingiococcus
- Laminicoccus
- Lankacoccus
- Lantanacoccus
- Lenania
- Leptococcus
- Leptorhizoecus
- Liucoccus
- Lomatococcus
- Londiania
- Longicoccus
- Maconellicoccus
- Macrocepicoccus
- Maculicoccus
- Madacanthococcus
- Madagasia
- Madangiacoccus
- Madeurycoccus
- Malaicoccus
- Malekoccus
- Mammicoccus
- Marendellea
- Mascarenococcus
- Maskellococcus
- Mauricoccus
- Melanococcus
- Metadenopsis
- Metadenopus
- Miconicoccus
- Mirococcopsis
- Mirococcus
- Miscanthicoccus
- Misericoccus
- Mizococcus
- Mollicoccus
- Mombasinia
- Moystonia
- Mutabilicoccus
- Nairobia
- Natalensia
- Neochavesia
- Neoclavicoccus
- Neoripersia
- Neosimmondsia
- Neotrionymus
- Nesococcus
- Nesopedronia
- Nesticoccus
- Nipaecoccus
- Novonilacoccus
- Octococcus
- Odacoccus
- Ohiacoccus
- Oracella
- Orococcus
- Orstomicoccus
- Oxyacanthus
- Palaucoccus
- Palmicultor
- Paludicoccus
- Pandanicola
- Papuacoccus
- Paracoccus
- Paradiscococcus
- Paradoxococcus
- Paraferrisia
- Paramococcus
- Paramonostherium
- Paramyrmococcus
- Parapaludicoccus
- Parapedronia
- Paraputo
- Pararhodania
- Paratrionymus
- Parkermicus
- Paulianodes
- Pedrococcus
- Pedronia
- Peliococcopsis
- Peliococcus
- Pelionella
- Pellizzaricoccus
- Penthococcus
- Peridiococcus
- Phenacoccus
- Phyllococcus
- Pilococcus
- Planococcoides
- Planococcus
- Pleistocerarius
- Plotococcus
- Poecilococcus
- Polystomophora
- Porisaccus
- Porococcus
- Prorhizoecus
- Prorsococcus
- Pseudantonina
- Pseudococcus
- Pseudorhizoecus
- Pseudorhodania
- Pseudoripersia
- Pseudotrionymus
- Puto
- Pygmaeococcus
- Quadrigallicoccus
- Rastrococcus
- Renicaula
- Rhizoecus
- Rhodania
- Ripersia
- Ritsemia
- Rosebankia
- Saccharicoccus
- Sarococcus
- Scaptococcus
- Seabrina
- Serrolecanium
- Seyneria
- Spartinacoccus
- Sphaerococcus
- Spilococcus
- Spinococcus
- Stachycoccus
- Stemmatomerinx
- Stipacoccus
- Strandanna
- Stricklandina
- Strombococcus
- Synacanthococcus
- Syrmococcus
- Tangicoccus
- Tasmanicoccus
- Telocorys
- Tibetococcus
- Tomentocera
- Trabutina
- Tridiscus
- Trimerococcus
- Trionymus
- Trochiscococcus
- Turbinococcus
- Tylococcus
- Tympanococcus
- Ventrispina
- Villosicoccus
- Volvicoccus
- Vryburgia
- Xenococcus
- Yudnapinna
References
- "Pseudococcidae Heymons, 1915". Integrated Taxonomic Information System.
- Noe, Ronald (November 21, 2012). "Fire Ants Protect Mealybugs against Their Natural Enemies by Utilizing the Leaf Shelters Constructed by the Leaf Roller Sylepta derogata". PLOS ONE. US National Library of Medicine National Institutes of Health. 7 (11): e49982. Bibcode:2012PLoSO...749982Z. doi:10.1371/journal.pone.0049982. PMC 3503828. PMID 23185505.
- Sharma, A.; Pati, P. K. (2013). "First record of Ashwagandha as a new host to the invasive mealybug (Phenacoccus solenopsis Tinsley) in India". Entomological News. 123 (1): 59–62. doi:10.3157/021.123.0114.
- Johnson, M.S.; et al. (2001). "Acropyga and Azteca Ants (Hymenoptera: Formicidae) with Scale Insects (Sternorrhyncha: Coccoidea): 20 Million Years of Intimate Symbiosis". American Museum Novitates. 3335: 1–18. doi:10.1206/0003-0082(2001)335<0001:AAAAHF>2.0.CO;2.
Further reading
- Jahn, G. C. and J. W. Beardsley (1994). "Big-headed ants, Pheidole megacephala: Interference with the biological control of gray pineapple mealybugs". In D.F. Williams [ed.] Exotic Ants: Biology, Impact and Control of Introduced Species. Boulder, Col.: Westview Press, 199–205. ISBN 9780813386157.
- Jahn, G. C. and J. W. Beardsley (1998). "Presence/absence sampling of mealybugs, ants, and major predators in pineapple". J. Plant Protection in the Tropics 11(1):73–79.
- Jahn, Gary C., J. W. Beardsley, and H. González-Hernández (2003). "A review of the association of ants with mealybug wilt disease of pineapple". Proceedings of the Hawaiian Entomological Society. 36:9–28.
External links
- NCIPM (National Centre for Integrated Pest Management) – Cotton Mealybugs
- Mealy bugs Pseudococcus spp.—BBC gardening advice
- CISR – Vine Mealybug—Center for Invasive Species Research summary on Vine Mealybug
- On the University of Florida / Institute of Food and Agricultural Sciences Featured Creatures website: