Vanadium(V) oxide
Vanadium(V) oxide (vanadia) is the inorganic compound with the formula V2O5. Commonly known as vanadium pentoxide, it is a brown/yellow solid, although when freshly precipitated from aqueous solution, its colour is deep orange. Because of its high oxidation state, it is both an amphoteric oxide and an oxidizing agent. From the industrial perspective, it is the most important compound of vanadium, being the principal precursor to alloys of vanadium and is a widely used industrial catalyst.[6]
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Divanadium pentaoxide | |
| Other names
Vanadium pentoxide Vanadic anhydride Divanadium pentoxide | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.013.855 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 2862 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties[1] | |
| V2O5 | |
| Molar mass | 181.8800 g/mol |
| Appearance | Yellow solid |
| Density | 3.357 g/cm3 |
| Melting point | 690 °C (1,274 °F; 963 K) |
| Boiling point | 1,750 °C (3,180 °F; 2,020 K) (decomposes) |
| 8.0 g/L (20 °C) | |
| +128.0·10−6 cm3/mol | |
| Structure[2] | |
| Orthorhombic | |
| Pmmn, No. 59 | |
a = 1151 pm, b = 355.9 pm, c = 437.1 pm | |
| Distorted trigonal bipyramidal (V) | |
| Thermochemistry | |
Std molar entropy (S |
130.54 J/mol·K [3] |
Std enthalpy of formation (ΔfH⦵298) |
-1550.590 kJ/mol [3] |
Gibbs free energy (ΔfG˚) |
-1419.435 kJ/mol [3] |
| Hazards | |
| Safety data sheet | ICSC 0596 |
| GHS pictograms |     |
| GHS Signal word | Danger |
GHS hazard statements |
H341, H361, H372, H332, H302, H335, H411 |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
10 mg/kg (rat, oral) 23 mg/kg (mouse, oral)[4] |
LCLo (lowest published) |
500 mg/m3 (cat, 23 min) 70 mg/m3 (rat, 2 hr)[4] |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
C 0.5 mg V2O5/m3 (resp) (solid)[5]
|
| Related compounds | |
Other anions |
Vanadium oxytrichloride |
Other cations |
Niobium(V) oxide Tantalum(V) oxide |
| Vanadium(II) oxide Vanadium(III) oxide Vanadium(IV) oxide | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
The mineral form of this compound, shcherbinaite, is extremely rare, almost always found among fumaroles. A mineral trihydrate, V2O5·3H2O, is also known under the name of navajoite.
Chemical properties
Reduction to lower oxides
Upon heating a mixture of vanadium(V) oxide and vanadium(III) oxide, comproportionation occurs to give vanadium(IV) oxide, as a deep-blue solid:[7]
- V2O5 + V2O3 → 4 VO2
The reduction can also be effected by oxalic acid, carbon monoxide, and sulfur dioxide. Further reduction using hydrogen or excess CO can lead to complex mixtures of oxides such as V4O7 and V5O9 before black V2O3 is reached.
Acid-base reactions
V2O5 is an amphoteric oxide. Unlike most metal oxides, it dissolves slightly in water to give a pale yellow, acidic solution. Thus V2O5 reacts with strong non-reducing acids to form solutions containing the pale yellow salts containing dioxovanadium(V) centers:
- V2O5 + 2 HNO3 → 2 VO2(NO3) + H2O
It also reacts with strong alkali to form polyoxovanadates, which have a complex structure that depends on pH.[8] If excess aqueous sodium hydroxide is used, the product is a colourless salt, sodium orthovanadate, Na3VO4. If acid is slowly added to a solution of Na3VO4, the colour gradually deepens through orange to red before brown hydrated V2O5 precipitates around pH 2. These solutions contain mainly the ions HVO42− and V2O74− between pH 9 and pH 13, but below pH 9 more exotic species such as V4O124− and HV10O285− (decavanadate) predominate.
Upon treatment with thionyl chloride, it converts to the volatile liquid vanadium oxychloride, VOCl3:[9]
- V2O5 + 3 SOCl2 → 2 VOCl3 + 3 SO2
Other redox reactions
Hydrochloric acid and hydrobromic acid are oxidised to the corresponding halogen, e.g.,
- V2O5 + 6HCl + 7H2O → 2[VO(H2O)5]2+ + 4Cl− + Cl2
Vanadates or vanadyl compounds in acid solution are reduced by zinc amalgam through the colourful pathway:
[[File:Vanadium oxidation states.jpg|none|300px|thumb| {{right| → → → [10] ]] The ions are all hydrated to varying degrees.
Preparation


Technical grade V2O5 is produced as a black powder used for the production of vanadium metal and ferrovanadium.[8] A vanadium ore or vanadium-rich residue is treated with sodium carbonate and an ammonium salt to produce sodium metavanadate, NaVO3. This material is then acidified to pH 2–3 using H2SO4 to yield a precipitate of "red cake" (see above). The red cake is then melted at 690 °C to produce the crude V2O5.
Vanadium(V) oxide is produced when vanadium metal is heated with excess oxygen, but this product is contaminated with other, lower oxides. A more satisfactory laboratory preparation involves the decomposition of ammonium metavanadate at 500-550 °C:[11]
- 2 NH4VO3 → V2O5 + 2 NH3 + H2O
Uses
Ferrovanadium production
In terms of quantity, the dominant use for vanadium(V) oxide is in the production of ferrovanadium (see above). The oxide is heated with scrap iron and ferrosilicon, with lime added to form a calcium silicate slag. Aluminium may also be used, producing the iron-vanadium alloy along with alumina as a by-product.[8]
Sulfuric acid production
Another important use of vanadium(V) oxide is in the manufacture of sulfuric acid, an important industrial chemical with an annual worldwide production of 165 million metric tons in 2001, with an approximate value of US$8 billion. Vanadium(V) oxide serves the crucial purpose of catalysing the mildly exothermic oxidation of sulfur dioxide to sulfur trioxide by air in the contact process:
- 2 SO2 + O2 ⇌ 2 SO3
The discovery of this simple reaction, for which V2O5 is the most effective catalyst, allowed sulfuric acid to become the cheap commodity chemical it is today. The reaction is performed between 400 and 620 °C; below 400 °C the V2O5 is inactive as a catalyst, and above 620 °C it begins to break down. Since it is known that V2O5 can be reduced to VO2 by SO2, one likely catalytic cycle is as follows:
- SO2 + V2O5 → SO3 + 2VO2
followed by
- 2VO2 +½O2 → V2O5
It is also used as catalyst in the selective catalytic reduction (SCR) of NOx emissions in some power plants. Due to its effectiveness in converting sulfur dioxide into sulfur trioxide, and thereby sulfuric acid, special care must be taken with the operating temperatures and placement of a power plant's SCR unit when firing sulfur-containing fuels.
Other oxidations
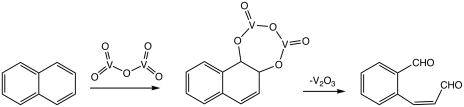
Maleic anhydride is produced by the V2O5-catalysed oxidation of butane with air:
- C4H10 + 4 O2 → C2H2(CO)2O + 8 H2O
Maleic anhydride is used for the production of polyester resins and alkyd resins.[13]
Phthalic anhydride is produced similarly by V2O5-catalysed oxidation of ortho-xylene or naphthalene at 350–400 °C. The equation is for the xylene oxidation:
- C6H4(CH3)2 + 3 O2 → C6H4(CO)2O + 3 H2O
Phthalic anhydride is a precursor to plasticisers, used for conferring pliability to polymers.
A variety of other industrial compounds are produced similarly, including adipic acid, acrylic acid, oxalic acid, and anthraquinone.[6]
Other applications
Due to its high coefficient of thermal resistance, vanadium(V) oxide finds use as a detector material in bolometers and microbolometer arrays for thermal imaging. It also finds application as an ethanol sensor in ppm levels (up to 0.1 ppm).
Vanadium redox batteries are a type of flow battery used for energy storage, including large power facilities such as wind farms.[14]
Biological activity

Vanadium(V) oxide exhibits very modest acute toxicity to humans, with an LD50 of about 470 mg/kg. The greater hazard is with inhalation of the dust, where the LD50 ranges from 4–11 mg/kg for a 14-day exposure.[6] Vanadate (VO3−
4), formed by hydrolysis of V2O5 at high pH, appears to inhibit enzymes that process phosphate (PO43−). However the mode of action remains elusive.[8]
References
- Weast, Robert C., ed. (1981). CRC Handbook of Chemistry and Physics (62nd ed.). Boca Raton, FL: CRC Press. p. B-162. ISBN 0-8493-0462-8..
- Shklover, V.; Haibach, T.; Ried, F.; Nesper, R.; Novak, P. (1996), "Crystal structure of the product of Mg2+ insertion into V2O5 single crystals", J. Solid State Chem., 123 (2): 317–23, doi:10.1006/jssc.1996.0186.
- R. Robie, B. Hemingway, and J. Fisher, “Thermodynamic Properties of Minerals and Related Substances at 298.15K and 1bar Pressure and at Higher Temperatures,” US Geol. Surv., vol. 1452, 1978.
- "Vanadium dust". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- NIOSH Pocket Guide to Chemical Hazards. "#0653". National Institute for Occupational Safety and Health (NIOSH).
- Günter Bauer, Volker Güther, Hans Hess, Andreas Otto, Oskar Roidl, Heinz Roller, Siegfried Sattelberger "Vanadium and Vanadium Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim. doi:10.1002/14356007.a27_367
- G. Brauer (1963). "Vanadium, Niobium, Tantalum". In G. Brauer (ed.). Handbook of Preparative Inorganic Chemistry, 2nd Ed. NY: Academic Press. p. 1267.
- Greenwood, Norman N.; Earnshaw, Alan (1984). Chemistry of the Elements. Oxford: Pergamon Press. pp. 1140, 1144. ISBN 978-0-08-022057-4..
- G. Brauer (1963). "Vanadium, Niobium, Tantalum". In G. Brauer (ed.). Handbook of Preparative Inorganic Chemistry, 2nd Ed. NY: Academic Press. p. 1264.
- "The oxidation states of vanadium". RSC Education. Retrieved 2019-10-04.
- G. Brauer (1963). "Vanadium, Niobium, Tantalum". In G. Brauer (ed.). Handbook of Preparative Inorganic Chemistry, 2nd Ed. NY: Academic Press. p. 1269.
- "Gibbs-Wohl Naphthalene Oxidation". Comprehensive Organic Name Reactions and Reagents. 2010. doi:10.1002/9780470638859.conrr270. ISBN 9780470638859.
- Tedder, J. M.; Nechvatal, A.; Tubb, A. H., eds. (1975), Basic Organic Chemistry: Part 5, Industrial Products, Chichester, UK: John Wiley & Sons.
- REDT Energy Storage. "Using VRFB for Renewable applications".
Further reading
- "Vanadium Pentoxide", Cobalt in Hard Metals and Cobalt Sulfate, Gallium Arsenide, Indium Phosphide and Vanadium Pentoxide (PDF), IARC Monographs on the Evaluation of Carcinogenic Risks to Humans 86, Lyon, France: International Agency for Research on Cancer, 2006, pp. 227–92, ISBN 92-832-1286-X.
- Vaidhyanathan, B.; Balaji, K.; Rao, K. J. (1998), "Microwave-Assisted Solid-State Synthesis of Oxide Ion Conducting Stabilized Bismuth Vanadate Phases", Chem. Mater., 10 (11): 3400–4, doi:10.1021/cm980092f.
External links
| Wikimedia Commons has media related to Vanadium(V) oxide. |
- How Vanadium Oxide Is Used In Energy Storage
- International Chemical Safety Card 0596
- NIOSH Pocket Guide to Chemical Hazards. "#0653". National Institute for Occupational Safety and Health (NIOSH).
- NIOSH Pocket Guide to Chemical Hazards. "#0654". National Institute for Occupational Safety and Health (NIOSH).
- Vanadium Pentoxide and other Inorganic Vanadium Compounds (Concise International Chemical Assessment Document 29)
- IPCS Environmental Health Criteria 81: Vanadium
- IPCS Health and Safety Guide 042: Vanadium and some vanadium salts
