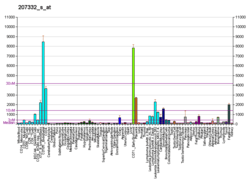
Transferrin receptor 1
Transferrin receptor protein 1 (TfR1), also known as Cluster of Differentiation 71 (CD71), is a protein that in humans is encoded by the TFRC gene.[5][6] TfR1 is required for iron import from transferrin into cells by endocytosis.[7][8]
Structure and function
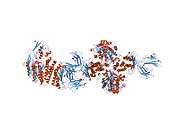
TfR1 is a transmembrane glycoprotein composed of two disulfide-linked monomers joined by two disulfide bonds. Each monomer binds one holo-transferrin molecule creating an iron-Tf-TfR complex which enters the cell by endocytosis.[9]
Clinical significance
TfR1 as a potential new target in cases of human leukemia & lymphoma. InatherYs, in Évry, France, developed a candidate drug, INA01 antibody (anti-CD71) that showed efficacy in pre-clinical studies in the therapy of two incurable orphan oncohematological diseases: the adult T cell leukemia (ATLL) caused by HTLV-1 and the Mantle cell lymphoma (MCL).
TfR1 expressed on the endothelial cells of the blood-brain barrier is used also in preclinical research to allow the delivery of large molecules including antibodies into the brain.[10] Some of the TfR1 targeting antibodies have been shown to cross the blood-brain barrier, without interfering with the uptake of iron. Amongst those are the mouse anti rat-TfR antibody OX26[11] and the rat anti mouse-TfR antibody 8D3.[12] The affinity of the antibody-TfR interaction seems to be important determining the success of transcytotic transport over endothelial cells of the BBB. Monovalent TfR interaction favors BBB transport due to altered intracellular sorting pathways. Avidity effects of bivalent interactions redirecting transport to the lysosome [13]. Also, reducing TfR binding affinity directly promote dissociation from the TfR which increase brain parenchymal exposure of the TfR binding antibody [14].
[1] Pardridge, Buciak, and Friden, “Selective Transport of an Anti-Transferrin Receptor Antibody through the Blood-Brain Barrier in Vivo.”
Interactions
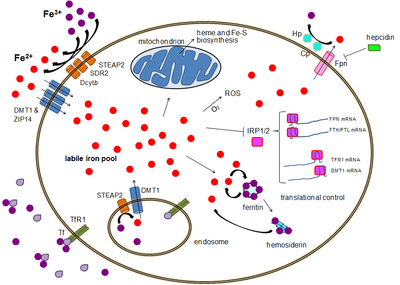
TfR1 has been shown to interact with GABARAP[15] and HFE.[16][17]
References
- GRCh38: Ensembl release 89: ENSG00000072274 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000022797 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Sutherland R, Delia D, Schneider C, Newman R, Kemshead J, Greaves M (July 1981). "Ubiquitous cell-surface glycoprotein on tumor cells is proliferation-associated receptor for transferrin". Proceedings of the National Academy of Sciences of the United States of America. 78 (7): 4515–9. doi:10.1073/pnas.78.7.4515. PMC 319822. PMID 6270680.
- Rabin M, McClelland A, Kühn L, Ruddle FH (November 1985). "Regional localization of the human transferrin receptor gene to 3q26.2----qter". American Journal of Human Genetics. 37 (6): 1112–6. PMC 1684729. PMID 3002171.
- Aisen P (November 2004). "Transferrin receptor 1". The International Journal of Biochemistry & Cell Biology. 36 (11): 2137–43. doi:10.1016/j.biocel.2004.02.007. PMID 15313461.
- Moos T (November 2002). "Brain iron homeostasis". Danish Medical Bulletin. 49 (4): 279–301. PMID 12553165.
- Speeckaert MM, Speeckaert R, Delanghe JR (December 2010). "Biological and clinical aspects of soluble transferrin receptor". Critical Reviews in Clinical Laboratory Sciences. 47 (5–6): 213–28. doi:10.3109/10408363.2010.550461. PMID 21391831.
- Johnsen KB, Burkhart A, Thomsen LB, Andresen TL, Moos T (October 2019). "Targeting the transferrin receptor for brain drug delivery". Progress in Neurobiology. 181: 101665. doi:10.1016/j.pneurobio.2019.101665. PMID 31376426.
- Pardridge WM, Buciak JL, Friden PM (October 1991). "Selective transport of an anti-transferrin receptor antibody through the blood-brain barrier in vivo". The Journal of Pharmacology and Experimental Therapeutics. 259 (1): 66–70. PMID 1920136.
- Lee HJ, Engelhardt B, Lesley J, Bickel U, Pardridge WM (March 2000). "Targeting rat anti-mouse transferrin receptor monoclonal antibodies through blood-brain barrier in mouse". The Journal of Pharmacology and Experimental Therapeutics. 292 (3): 1048–52. PMID 10688622.
- Niewoehner J, Bohrmann B, Collin L, Urich E, Sade H, Maier P, et al. (January 2014). "Increased brain penetration and potency of a therapeutic antibody using a monovalent molecular shuttle". Neuron. 81 (1): 49–60. doi:10.1016/j.neuron.2013.10.061. PMID 24411731.
- Yu YJ, Zhang Y, Kenrick M, Hoyte K, Luk W, Lu Y, et al. (May 2011). "Boosting brain uptake of a therapeutic antibody by reducing its affinity for a transcytosis target". Science Translational Medicine. 3 (84): 84ra44. doi:10.1126/scitranslmed.3002230. PMID 21613623.
- Green F, O'Hare T, Blackwell A, Enns CA (May 2002). "Association of human transferrin receptor with GABARAP" (PDF). FEBS Letters. 518 (1–3): 101–6. doi:10.1016/S0014-5793(02)02655-8. PMID 11997026.
- Feder JN, Penny DM, Irrinki A, Lee VK, Lebrón JA, Watson N, et al. (February 1998). "The hemochromatosis gene product complexes with the transferrin receptor and lowers its affinity for ligand binding". Proceedings of the National Academy of Sciences of the United States of America. 95 (4): 1472–7. doi:10.1073/pnas.95.4.1472. PMC 19050. PMID 9465039.
- West AP, Bennett MJ, Sellers VM, Andrews NC, Enns CA, Bjorkman PJ (December 2000). "Comparison of the interactions of transferrin receptor and transferrin receptor 2 with transferrin and the hereditary hemochromatosis protein HFE". The Journal of Biological Chemistry. 275 (49): 38135–8. doi:10.1074/jbc.C000664200. PMID 11027676.