Paleobiota of the Ciechocinek Formation
The Ciechocinek Formation, is a Jurassic (lower to middle Toarcian) geologic formation that extends from Grimmen, Germany, to Poland.[1] Dinosaur remains are among the fossils that have been recovered from the formation, including the Thyreorporan Emausaurus and others which have not yet been referred to a specific genus. On Poland, the main basin if charaserized mostly by lack of marine microfauna in the Częstochowa-Zawiercie area. It is mainly composed by Foraminiferans: Ammodiscus glumaceous, Trochammina? sp., Lagenammina sp., Saccammina? sp. and Dentalina sp. (On Pabianice, Łutowiec and Żarki), as well as the Ostracodan Liasina sp.[2] The crustacean Estheria is relatively abundant, along traces of Diplocraterion and the inconclusive remains of Clams, Snails and Fish teeth (On Żuki, Gorzków, Choroń and Bobolice). The discussed settlement complex, due to its characteristic lithological education with floristic and faunistic analogies, allows without difficulty to parallelize with similarly developed sediments in the Polish Lowlands.[3]

| Part of a series on |
| Paleontology |
|---|
 |
|
Fossils
|
|
Natural history |
|
Organs and processes
|
|
Evolution
|
|
History of paleontology |
|
Branches of paleontology |
|
Paleontology Portal Category |
Dinoflagellates
Color key
|
Notes Uncertain or tentative taxa are in small text; |
| Genus | Species | Location | Material | Notes | Images |
|---|---|---|---|---|---|
|
Nannoceratopsis[4] |
|
|
Dinocysts |
A Dinophyceae Dinoflagellatan, type member of the family Nannoceratopsiaceae. The large amount of Cysts of the genus point to more diversified marine palaeoenvironments. N. senex is the most abundant. |
|
|
Luehndea[4] |
|
|
Dinocysts |
A Dinophyceae Dinoflagellatan, type member of the family Luehndeoideae. Presence of Luehndea spinosa suggests Late Pliensbachian–earliest Toarcian age of studied assemblages. |
|
Fungi
| Genus | Species | Location | Material | Notes | Images |
|---|---|---|---|---|---|
|
Indeterminate |
|
Fungal spores of different sizes associated with wood |
Associated with a high rate of organic burial, the presence of Fungal Matter increased on the Uppermost layers of the Drzewica Formation, with a continue deposition between the T-OAE extincion, and several ups and downs on the Ciechocinek Strata, related with local climate and humidity changes. This is rather a reflection of the efficiency of terrestrial biodegradation.[5] Measured increasing of temperature favoured local fungal-mediated decomposition of plant litter, specifically of normally resistant wood.[5] |
Xylophagous Fungi like Fomitopsis are probably the main origin for the Fungal Spores found on the Ciechocinek Formation | |
|
Sporonites[6] |
|
|
Spores |
Fungal Spores with non diagnosed affinities, probably related with Pucciniomycetes inside Agaricomycotina. It is found associated with Pollen and Spores, interpreted as some sort of parasitism. |
 Sporonites was probably related with plant parasite fungus, such as Gymnosporangium |
Invertebrata
Ichnofossils
| Genus | Species | Location | Material | Notes | Images |
|---|---|---|---|---|---|
|
|
Burrowing and track ichnofossils. |
Burrow-like ichnofossils. Planolites is really common in all types of the Ciechocinek Formation deposits. It is referred to vermiform deposit-feeders, mainly Polychaetes, producing active Fodinichnia. It is controversial, since is considered a strictly a junior synonym of Palaeophycus.[8] |
 Example of Planolites fossil | |
|
Palaeophycus[7] |
|
|
Burrowing and track ichnofossils. |
Burrow-like ichnofossils. Palaeophycus is less common than Planolites in deposits of the Ciechocinek Formation. On the Kozlowice outcrop however there are numerous specimens occur, interpreted as the result of passive filling of polychaete burrows.[7] |
Example of Palaeophycus fossil |
|
|
Burrowing and track ichnofossils. |
Burrow-like ichnofossils. There are a few specimens in the Kozlowice outcrop. It is interpreted as a grazing trail or Fodinichnia, produced at shallow depth in sediment by Polychaetes and Priapulids.[7] |
Example of Helminthopsis fossil | |
|
Gyrochorte[7] |
|
|
Burrowing and track ichnofossils. |
Burrow-like ichnofossils. Gyrochorte is interpreted as a result of active digging on the sediment by deposit-feeding worm-like animal, probably an Annelid or similar kinds of creatures.[7] On the Kozlowice strata only a few specimens where observed. |
|
|
Protovirgularia[7] |
|
|
Bilobate trace fossil |
Bottom Trace Fossils. Protovirgularia is a Repichnia form, ascribed to the activity of Bivalves, leaving a trace due to the rhythmic action of a foot.[7] |
|
|
Spongeliomorpha[7] |
|
|
Burrows and associated traces |
Burrow-like ichnofossils. Spongeliomorpha is believed to come from the domicile of Crustaceans: Anomuras (Probably Eocarcinoidea) and Decapodas (Probably Glypheidae), created as they dig in a firm, semiconsolidated substrate.[7] Local Spongeliomorpha could point to a transgressional Sea water impulse following a short episode of regression.[7] |
|
|
|
Burrows and associated traces |
Burrow-like ichnofossils. Most Diplocraterion show only protrusive spreit, like the local ones, produced under predominantly erosive conditions where the organism was constantly burrowing deeper into the substrate as sediment was eroded from the top. It can be Made by crustaceans, annelids or other benthic fauna.[7] |
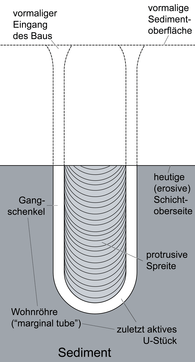 Diplocraterion parallelum diagram | |
Annelida
| Genus | Species | Stratigraphic position | Material | Notes | Images |
|---|---|---|---|---|---|
|
Dictyothylakos[9] |
|
|
Leech cocoons |
Hirudinea cocoons, identified with palynological residues. The cocoons Dictyothylakos are common on flooded basin sediments, and implies not only the presence of parasitic leeches, but also the presence of large hosts nearby, that is proven on the case of Ciechocinek thanks to the dinosaur fossils from the German realm. |
 Example of Leech Cocoon |
Brachiopoda
| Genus | Species | Stratigraphic position | Material | Notes | Images |
|---|---|---|---|---|---|
|
|
Cunchs |
A Brachiopodan, member of Discinidae inside Discinida. The classification of the Discinidae is rather treated with confusion, due to the description and the identification of either extinct and extant genera and species. |
||
Bivalvia
| Genus | Species | Stratigraphic position | Material | Notes | Images |
|---|---|---|---|---|---|
|
|
Cunchs |
A Clam, member of Inoceramidae inside Myalinida. This genus resemble the Modern Pearl Oysters, although it was more likely a clam. The specimens are rather complete. |
||
|
|
Cunchs |
A Clam, member of Inoceramidae inside Myalinida. Pseudomytiloides dubius is a possible junior synonym. This genus resemble the Modern Pearl Oysters, although it was more likely a clam. The specimens are rather complete. It is the most common bivalve found locally. |
Parainoceramya | |
|
|
Cunchs |
A Clam, member of Lucinidae inside Lucinida. Very abundant on the layers |
| |
|
|
Cunchs |
A Clam, member of Pholadomyidae inside Pholadomyida. Rather common, but less abundant than other local genera |
||
|
|
Cunchs |
A Clam, member of Cuspidariidae inside Anomalodesmata. Marginal Marine to Mangrove swamp mollusc fauna, present on a rather large degree of salinities. |
||
Gastropoda
| Genus | Species | Stratigraphic position | Material | Notes | Images |
|---|---|---|---|---|---|
|
|
Cunchs |
A Sea Snail, member of Cerithiinae inside Caenogastropoda. The main recovered gasteropod from the Green Series. Extant genus. |
| |
Cephalopoda
| Genus | Species | Stratigraphic position | Material | Notes | Images |
|---|---|---|---|---|---|
|
|
Shells |
 Eleganticeras | ||
|
|
Shells |
| ||
|
|
Shells |
| ||
|
|
Shells |
A Phylloceratidae Ammonite. Among the largest ammonites found on the Green Series, with specimens over 60 cm |
| |
|
|
Shells |
| ||
|
|
Shells |
| ||
|
|
Shells |
 Pseudolioceras | ||
|
|
Shells |
A Dactylioceratinae Ammonite. Most common ammonite found on the Green Series and the different erratic boulders, as is the most common on the German realm, on the north and the south, with several specimens of different sizes. |
| |
|
|
Shells |
|||
|
|
Multiple Specimens. |
A Passaloteuthididae Belemnoidean. |
||
|
|
Multiple Specimens. |
A Megateuthididae Belemnoidean. |
||
|
|
Multiple Specimens. |
A Megateuthididae Belemnoidean. |
| |
|
|
Multiple Specimens. |
A Beloteuthidae Mesoteuthoidean. Is a relatively small genus. |
.jpg) Beloteuthis | |
|
|
Multiple Specimens. |
A Teudopsidae Vampyropodan. Related to the modern Vampyroteuthis infernalis. |
| |
|
|
Multiple Specimens. |
A Loligosepiidae Loligosepiidan (Vampyromorpha). Related to the modern Vampyroteuthis infernalis. Gladii of Loligosepia can be distinguished from Jeletzkyteuthis by the transition lateral field/hyperbolar zone. Described originally as Belopeltis bollensis. |
||
Echinodermata
| Genus | Species | Stratigraphic position | Material | Notes | Images |
|---|---|---|---|---|---|
|
|
Various specimens. |
An Ophiuroidean Echinodermatan. It is relatively less abundant than in coeval strata. |
 Palaeocoma | |
|
|
Various specimens. |
An Ophiodermatidae Echinodermatan. Still alive today. On the Green Series, is related to deep basinal deposits. |
| |
|
Archastropecten[23] |
|
|
Various specimens. |
An Astropectinidae Asteroidean. Related to freshwater debris, that probably caused changes to salinity and mass mortality of Echinoderms. |
 Archastropecten |
|
Chladocrinus[23] |
|
|
Multiple specimens. |
||
|
|
Spines from two Echinoidea Species |
Sea Urchin Echinodermatan. |
||
Crustacea
| Genus | Species | Stratigraphic position | Material | Notes | Images |
|---|---|---|---|---|---|
|
|
Various specimens. |
An Eocarcinoidean Anomuran. It is among the oldest found Crabs worldwide. The genus is known locally mostly due to fragmentary specimens, related to less saline conditions. |
||
|
|
Various specimens. |
A Glypheidae Decapodan. The most complete Crustacean found on the formation |
| |
|
Proeryon[25] |
|
|
Single specimen with preserved upper torax and chelae |
A Proeryoninae Polychelidan. |
 Proeryon |
|
|
Docens of Specimens |
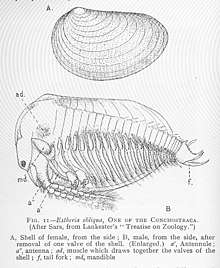
A Clam shrimp (Phyllopodan) of the family Lioestheriidae. They fed on detritus, being very small slow moving, nektonic organisms that filter fed as they floated. The presence of Lioestheria marks the appearance of less saline conditions, as this is a mostly freshwater genus. The local Phyllopods are related with a great amount of freshwater debris (specially plants), and suggest seasonal changes on the rivers on the Toarcian Polish Basin. |
| |
|
Liasina[26] |
|
|
Valves |
An Ostracodan of the family Pontocyprididae. Small marine ostracods related with abundant Green Algae environments |
|
|
Ambigocythere[27] |
|
|
Cunchs |
A Marine Ostracodan with incertade Sedis assignation. The specimens of this genus are rather fargmentary. |
|
|
Infracytheropteron[28] |
|
|
Cunchs |
A Marine Ostracodan of the family Protostomia. The specimens of this genus are rather fragmentary and of uncertain nature. |
|
|
|
Cunchs |
A Marine Ostracodan of the family Healdiidae inside Podocopida. This genus is the main reported on the marine facies of the Dobbertin Clay Pit. |
||
|
Ogmoconcha[32] |
|
|
Cunchs |
A Marine Ostracodan of the family Healdiidae inside Podocopida. Is probably present on all the Clay pits, altrougth the other locations specimens wheren´t published. |
|
|
Ledahia[33] |
|
|
Cunchs |
A Marine Ostracodan of the family Healdiidae inside Podocopida. One of the genus that reflect better the migration patterns of ostracodans on the Pliensbachian-Toarcian boundary. |
|
|
Pseudohealdia[33] |
|
|
Cunchs |
A Marine Ostracodan of the family Healdiidae inside Podocopida. The genus is rare on the layers. |
|
|
Hermiella[30] |
|
|
Cunchs |
A Marine Ostracodan of the family Healdiidae inside Podocopida. This genus is the main reported on the marine facies of the Dobbertin Clay Pit. |
|
|
|
Cunchs |
A Marine Ostracodan of the family Cytheruridae inside Podocopida. Is rare and the specimens found are rather incomplete. |
||
|
|
Cunchs |
A Marine Ostracodan of the family Cytheruridae inside Podocopida. The most abundant genus on the Grimmen Clay Pit. |
||
|
Procytherura[36] |
|
|
Cunchs |
A Marine Ostracodan of the family Cytheruridae inside Podocopida. A genus with well preserved specimens locally. |
|
|
|
Cunchs |
A Marine Ostracodan of the family Protocytheridae inside Podocopida. Common, and associated to benthonic deposits. This genus maybe was able to resist relative changes in salinity. |
||
|
Kinkelinella[27] |
|
|
Cunchs |
A Marine Ostracodan of the family Protocytheridae inside Podocopida. A genus related with Fish fossils and anoxic bottoms. |
|
|
|
Cunchs |
A Marine Ostracodan of the family Bairdiidae inside Bairdioidea. Abundant and diverse, is found associated with Ammonite shells. |
||
|
|
Cunchs |
A Marine Ostracodan of the family Bairdiidae inside Bairdioidea. Less abundant than the genus Bairdia, is present on layers where Wood debris is more abundant. |
||
|
|
Cunchs |
A Marine Ostracodan of the family Bairdiidae inside Bairdioidea. Know only from the Green Series, is a rather unusual and complex genus. |
||
|
|
Cunchs |
A Marine Ostracodan of the family Bairdiidae inside Bairdioidea. It is a relatively abundant genus, but based on incomplete material. |
||
|
Polycope[28] |
|
|
Cunchs |
A Marine Ostracodan of the family Polycopidae inside Cladocopina. Scarce but well preserved specimens. |
|
Arachnida
| Genus | Species | Stratigraphic position | Material | Notes | Images |
|---|---|---|---|---|---|
|
|
Single Incomplete Specimen. |
A possible member of the superfamily Palpimanoidea.[41] It is the first confirmed spider from the lower jurassic, and a rare find, probably washed to the sea due to a hard wind related to hurricane action, present due to the measured moonsonal conditions of the formation, as on the rest of the Lower-Middle Toarcian strata. Probably a ground dewelling predator that hunted the abundant insect fauna present on the layers.[41] With a robust and well-armed legs I, directed forwards give the suggestion that they were preycapture appendages, a morphology typical of a sit-and-wait predator, while the short legs III are more typical on web spiders, especially Orbweavers, but also found on Palpimanoids, but not on that that are common substrate dwellers, that had legs more equal.[41] Seppo was probably not a habitual ground dweller, with armoured front legs related to capturing dangerous prey, such as many palpimanoids today are Araneophagous, for example.[41] |
 Seppo koponeni reconstruction on a Tree, as suggested for it's Leg III morphology | |
Insecta
Insects are a common terrestrial animals that where proabaly drifted to the sea due to Moonsonal conditions present on the Ciechocinek Formation.[42]
| Genus | Species | Stratigraphic position | Material | Notes | Images |
|---|---|---|---|---|---|
|
|
Wing Scales |
A Lepidoptera (Moth) of the family Eolepidopterigidae. They are essential fossils for the Development of the color on Lepidopterans. |
||
|
Zalmonites[44] |
|
|
Several Specimens |
A Grasshopper. |
|
|
|
Several Specimens |
A Grasshopper of the family Elcanidae. The species P. magna is among the largest Orthopterans of the Jurassic, while P. minima is among the Smallest. |
||
|
|
Several Specimens |
A Grasshopper of the family Elcanidae. |
||
|
|
Several Specimens |
A Grasshopper of the family Elcanidae. |
||
|
|
Several Specimens |
A Grasshopper of the family Elcanidae. |
||
|
|
Several Specimens |
A Grasshopper of the family Locustopsidae. |
||
|
|
Several Specimens |
A Grasshopper of the family Locustopsidae. |
||
|
|
Several Specimens |
A Grasshopper of the family Locustopsidae. |
||
|
|
Several Specimens |
A Grasshopper of the family Locustidae. |
||
|
|
Several Specimens |
A Grasshopper of the family Regiatidae. |
||
|
|
Several Specimens |
A Cricket of the family Protogryllidae. |
||
|
Griphopteron[50] |
|
|
Several Specimens |
A Cricket of the family Blattogryllidae. |
|
|
|
Several Specimens |
A Stick Insect of the family Aerophasmidae. |
||
|
Aenne[51] |
|
|
Wings |
The oldest known non biting midge (Chironomidae) |
|
|
Dobbertiniopteryx[52] |
|
|
Wings |
A Stonefly of the family Protostomia |
|
|
|
Several Specimens |
A Crane Fly of the family Limoniidae. |
||
|
|
Several Specimens |
A Crane Fly of the family Limoniidae. A. nana can be the smallest Crane fly of the Jurassic. |
||
|
Eotipula[44] |
|
|
Several Specimens |
A Crane Fly of the family Limoniidae. |
|
|
Phryganidium[44] |
|
|
Several Specimens |
A Crane Fly of the family Limoniidae. |
|
|
Grimmenia[53] |
|
|
Several Specimens |
A Crane Fly of the family Limoniidae. |
|
|
Rhaetomyia[50] |
|
|
Several Specimens |
A Phantom midge of the family Chaoboridae. |
|
|
Grimmyia[50] |
|
|
Several Specimens |
A Snipe Fly of the family Rhagionidae. |
|
|
Liassobrachyceron[55] |
|
|
Several Specimens |
A Snipe Fly of the family Rhagionidae. |
|
|
Palaeobrachyceron[50] |
|
|
Several Specimens |
A Snipe Fly of the family Rhagionidae. |
|
|
Antefungivora[50] |
|
|
Several Specimens |
A Fly of the family Antefungivoridae. |
|
|
Archibio[50] |
|
|
Several Specimens |
A Fly of the family Antefungivoridae. |
|
|
Pleciofungivora[50] |
|
|
Several Specimens |
A Fly of the family Pleciofungivoridae. |
|
|
Archirhyphus[50] |
|
|
Several Specimens |
A Fly of the family Protorhyphidae. |
|
|
Protorhyphus[47] |
|
|
Several Specimens |
A Fly of the family Protorhyphidae. |
|
|
Protobrachyceron[56] |
|
|
Several Specimens |
A Fly of the family Protobrachyceridae. |
|
|
Heterorhyphus[50] |
|
|
Several Specimens |
A Fly of the family Heterorhyphidae. |
|
|
Eoditomyia[50] |
|
|
Several Specimens |
A Fly of the family Eoditomyidae. |
|
|
Archipleciomima[50] |
|
|
Several Specimens |
A Fly. |
|
|
Protoplecia[50] |
|
|
Several Specimens |
A Fly of the family Protopleciidae. |
|
|
Mailotrichocera[57] |
|
|
Several Specimens |
A Winter Crane Fly of the family Trichoceridae. |
|
|
Nannotanyderus[58] |
|
|
Several Specimens |
A primitive Crane Fly of the family Tanyderidae. |
|
|
Eoptychoptera[47] |
|
|
Several Specimens |
A Phantom crane fly of the family Ptychopteridae. |
|
|
Eolimnobia[47] |
|
|
Several Specimens |
A Phantom crane fly of the family Ptychopteridae. |
|
|
Tanypsycha[58] |
|
|
Several Specimens |
A Moth fly of the family Psychodidae. |
|
|
Liassopsychodina[58] |
|
|
Several Specimens |
A Moth fly of the family Psychodidae. |
|
|
Mesorhyphus[56] |
|
|
Several Specimens |
A Wood Gnat of the family Anisopodidae. |
|
|
Metatrichopteridium[59] |
|
|
Several Specimens |
A rare Fly of the family Hennigmatidae. |
|
|
Orthophlebia[47] |
|
|
Several Specimens |
A Mecopteran of the family Orthophlebiidae. |
 Orthophlebia fossil |
|
Neorthophlebia[47] |
|
|
Several Specimens |
A Mecopteran of the family Bittacidae. |
|
|
Parabittacus[60] |
|
|
Several Specimens |
A Mecopteran of the family Bittacidae. |
|
|
Mesobittacus[47] |
|
|
Several Specimens |
A Mecopteran of the family Bittacidae. |
|
|
Pseudopolycentropus[44] |
|
|
Several Specimens |
A Mecopteran of the family Pseudopolycentropodidae. |
|
|
Mesochorista?[44] |
|
|
Several Specimens |
A Mecopteran of the family Permochoristidae. |
|
|
Liasocoris[61] |
|
|
Several Specimens |
A Hemipteran. |
|
|
|
Several Specimens |
A Hemipteran of the family Fulgoridiidae. The colossal abundance of the genus maybe it's related to a preference for seashore habitats. Some specimens are indentinguible, making possible some species synonymous. |
||
|
Metafulgoridium[47] |
|
|
Several Specimens |
A Hemipteran of the family Fulgoridiidae. |
|
|
Archiconiopteryx[62] |
|
|
Several Specimens |
A Hemipteran of the family Archiconiopterygidae. |
|
|
Liadopsylla[63] |
|
|
Several Specimens |
A Hemipteran of the family Liadopsyllidae. |
|
|
Hadrocoris[56] |
|
|
Several Specimens |
A Hemipteran of the family Hadrocoridae. |
|
|
Progonocimex[56] |
|
|
Several Specimens |
A Hemipteran of the family Progonocimicidae. |
|
|
Eocercopis[47] |
|
|
Several Specimens |
A Hemipteran of the family Progonocimicidae. |
|
|
Archicercopis[47] |
|
|
Several Specimens |
A Hemipteran of the family Progonocimicidae. |
|
|
Archegocimex[44] |
|
|
Several Specimens |
A Hemipteran of the family Archegocimicidae. |
|
|
Progonocoris[56] |
|
|
Several Specimens |
A Hemipteran of the family Archegocimicidae. |
|
|
Anosmus[47] |
|
|
Several Specimens |
A Hemipteran of the family Archegocimicidae. |
|
|
Diatillus[63] |
|
|
Several Specimens |
A Hemipteran of the family Archegocimicidae. |
|
|
Pachymeridium[64] |
|
|
Several Specimens |
A Hemipteran of the family Pachymeridiidae. |
|
|
Sisyrocoris[63] |
|
|
Several Specimens |
A Hemipteran of the family Pachymeridiidae. |
|
|
Hypocimex[47] |
|
|
Several Specimens |
A Hemipteran of the family Pachymeridiidae. |
|
|
Apsicoria[47] |
|
|
Several Specimens |
A Hemipteran of the family Pachymeridiidae. |
|
|
Cathalus[47] |
|
|
Several Specimens |
A Hemipteran of the family Pachymeridiidae. |
|
|
Psychrocoris[56] |
|
|
Several Specimens |
A Hemipteran of the family Pachymeridiidae. |
|
|
Cuneocoris[56] |
|
|
Several Specimens |
A Hemipteran of the family Cuneocoridae. |
|
|
Apopnus[63] |
|
|
Several Specimens |
A Hemipteran of the family Naucoridae. |
|
|
Aphlebocoris[44] |
|
|
Several Specimens |
A Hemipteran of the family Naucoridae. |
|
|
|
Several Specimens |
A Hemipteran of the family Archijassidae. |
||
|
|
Several Specimens |
A Hemipteran of the family Archijassidae. |
||
|
|
Several Specimens |
A Hemipteran of the family Hylicellidae. |
||
|
|
Several Specimens |
A Hemipteran of the family Protopsyllidiidae. |
||
|
|
Several Specimens |
A Hemipteran of the family Oviparosiphidae. |
||
|
Grimmaratavites[66] |
|
|
Several Specimens |
A Hemipteran of the family Karatavitidae. |
|
|
Liassochrysa[67] |
|
|
Several Specimens |
A Neuropteran of the family Mantispidae. It is the earliest know Chrysopid |
|
|
Prohemerobius[68] |
|
|
Several Specimens |
A Neuropteran of the family Prohemerobiidae. |
_(16041759264).jpg) Prohemerobius at the bottom |
|
Mesosmylina[69] |
|
|
Several Specimens |
A Neuropteran of the family Osmylidae. |
|
|
Polyosmylus[50] |
|
|
Several Specimens |
A Neuropteran. |
|
|
Mesoleon[50] |
|
|
Several Specimens |
A Neuropteran. |
|
|
|
Several Specimens |
A Neuropteran. |
||
|
|
Several Specimens |
A Neuropteran. |
||
|
Melamnous[47] |
|
|
Several Specimens |
A Neuropteran. |
|
|
Solenoptilon[47] |
|
|
Several Specimens |
A Neuropteran of the Family Solenoptilidae. |
|
|
Epigambria[47] |
|
|
Several Specimens |
A Neuropteran of the Family Epigambriidae. |
|
|
Apeirophlebia[47] |
|
|
Several Specimens |
A Neuropteran of the Family Psychopsidae. An unexpectec giant Silky lacewing, with a size up to 7 cm. |
|
|
|
Several Specimens |
An Odonatan. Very large dragonfly, with a wingspan of 13 cm |
||
|
Liadothemis[47] |
|
|
Several Specimens |
An Odonatan. |
|
|
Petrothemis[47] |
|
|
Several Specimens |
An Odonatan. |
|
|
Parelthothemis[47] |
|
|
Several Specimens |
An Odonatan. |
|
|
Anomothemis[47] |
|
|
Several Specimens |
An Odonatan. |
|
|
Rhabdothemis[47] |
|
|
Several Specimens |
An Odonatan. |
|
|
Temnostigma[47] |
|
|
Several Specimens |
An Odonatan. |
|
|
Pycnothemis[47] |
|
|
Several Specimens |
An Odonatan. |
|
|
Archithemis[47] |
|
|
Several Specimens |
An Odonatan of the family Archithemistidae. |
|
|
Selenothemis[47] |
|
|
Several Specimens |
An Odonatan of the family Selenothemistidae. |
|
|
Heterothemis[72] |
|
|
Several Specimens |
An Odonatan of the family Liassogomphidae. |
|
|
Anisozygopteron[47] |
|
|
Several Specimens |
An Odonatan of the family Myopophlebiidae. |
|
|
Dialothemis[73] |
|
|
Several Specimens |
An Odonatan of the family Selenothemistidae. |
|
|
Eosagrion[56] |
|
|
Several Specimens |
An Odonatan of the family Eosagrionidae. |
|
|
Turanopteron[50] |
|
|
Several Specimens |
An Odonatan of the family Asiopteridae. |
|
|
Liadobracona[74] |
|
|
Several Specimens |
A Hymenopteran of the family Ephialtitidae. |
|
|
Brigittepterus[75] |
|
|
Several Specimens |
A Hymenopteran of the family Ephialtitidae. |
|
|
Xyelula[75] |
|
|
Several Specimens |
A Hymenopteran of the family Sepulcidae. |
|
|
Mesoblattina[76] |
|
|
Several Specimens |
A Cockroach of the family Mesoblattinidae. |
|
|
|
Several Specimens |
A Cockroach of the family Caloblattinidae. |
||
|
|
Several Specimens |
A Cockroach of the family Raphidiomimidae. |
||
|
|
Several Specimens |
A Cockroach. Blattulidae is an extinct cockroach family which was widely distributed around the world and lasted from Late Triassic to Cretaceous. |
||
|
|
Several Specimens |
A Cockroach. Blattulidae is an extinct cockroach family which was widely distributed around the world and lasted from Late Triassic to Cretaceous. |
||
|
Dicronemoura[50] |
|
|
Several Specimens |
A Plecopteran of the family Perlariopseidae. |
|
|
Liassopsocus[50] |
|
|
Several Specimens |
A Permopsocidan of the family Psocidiidae. |
|
|
Archipsylla[50] |
|
|
Several Specimens |
A Permopsocidan of the family Archipsyllidae. |
|
|
Undacypha[50] |
|
|
Several Specimens |
A Dicondylian of uncertaing Placement. |
|
|
Parnidium[44] |
|
|
Several Specimens |
A Coleopteran. |
|
|
Polypamon[79] |
|
|
Several Specimens |
A Coleopteran. |
|
|
Bathygerus[79] |
|
|
Several Specimens |
A Coleopteran. |
|
|
Plastonebria[79] |
|
|
Several Specimens |
A Coleopteran. |
|
|
Plastobuprestites[79] |
|
|
Several Specimens |
A Coleopteran. |
|
|
Nannoodes[79] |
|
|
Several Specimens |
A Coleopteran. |
|
|
Pseudocyphon[79] |
|
|
Several Specimens |
A Coleopteran. |
|
|
Keleusticus[79] |
|
|
Several Specimens |
A Coleopteran. |
|
|
Allognosis[79] |
|
|
Several Specimens |
A Coleopteran. |
|
|
Enamma[79] |
|
|
Several Specimens |
A Coleopteran. |
|
|
Pseudoprionites[79] |
|
|
Several Specimens |
A Coleopteran. |
|
|
Nebrioides[79] |
|
|
Several Specimens |
A Coleopteran. |
|
|
Paracurculium[79] |
|
|
Several Specimens |
A Coleopteran. |
|
|
Apioderes[79] |
|
|
Several Specimens |
A Coleopteran. |
|
|
Bareus[79] |
|
|
Several Specimens |
A Coleopteran. |
|
|
Anypostatus[79] |
|
|
Several Specimens |
A Coleopteran. |
|
|
Periboloptera[79] |
|
|
Several Specimens |
A Coleopteran. |
|
|
Clinomerus[79] |
|
|
Several Specimens |
A Coleopteran. |
|
|
Anomerus[79] |
|
|
Several Specimens |
A Coleopteran. |
|
|
Masselytron[79] |
|
|
Several Specimens |
A Coleopteran. |
|
|
Oxytoroptera[79] |
|
|
Several Specimens |
A Coleopteran. |
|
|
Ecthlimma[47] |
|
|
Several Specimens |
A Coleopteran. |
|
|
Onkedodimus[47] |
|
|
Several Specimens |
A Coleopteran. |
|
|
Apsychus[47] |
|
|
Several Specimens |
A Coleopteran. |
|
|
|
Several Specimens |
A Coleopteran. |
||
|
Anancaeon[47] |
|
|
Several Specimens |
A Coleopteran. |
|
|
Hydrobiites[47] |
|
|
Several Specimens |
A Coleopteran of the family Permosynidae. |
|
|
Thoracotes[44] |
|
|
Several Specimens |
A Coleopteran of the family Trogossitidae. |
|
|
Eurynucha[44] |
|
|
Several Specimens |
A Coleopteran of the family Buprestidae. |
|
|
Coptogyrinus[44] |
|
|
Several Specimens |
A Coleopteran of the family Gyrinidae. |
|
|
Carabites[44] |
|
|
Several Specimens |
A Coleopteran of the family Carabidae. |
|
|
Nele[50] |
|
|
Several Specimens |
A Grylloblattodean of the family Bajanzhargalanidae. |
|
Vertebrata
Fishes
Actinopteri
| Genus | Species | Stratigraphic position | Material | Notes | Images |
|---|---|---|---|---|---|
|
|
Multiple specimens. |
The youngest representative of the family Saurichthyidae, known for its large jaws, similar to modern Belonidae. |
| |
|
Grimmenichthys[81] |
|
|
GG 439/1, articulated, but incompletely preserved specimen |
A member of the Pholidophoriformes. |
|
|
Pholidophoriformes[81] |
|
|
GG 439/2 |
Non assigned to an especific genus |
|
|
|
Fragmentary remains |
Non assigned to an especific species |
| |
|
|
Unknown, only cited. |
Non assigned to an especific genus |
||
|
|
|
Type member of the Leptolepidae. |
| |
|
Indeterminate |
|
|
Non assigned to a concrete genus. |
||
|
Proleptolepis sp. |
|
|
Member of the Leptolepidae. |
||
|
|
|
Member of the Semionotidae. |
| |
|
|
Various specimens |
Member of the Semionotidae. |
| |
|
|
Teeth & Scales |
Member of the Semionotidae. |
||
|
Grimmenodon[86] |
|
|
GG 437, Fragmentary Skull |
Member of the Pycnodontiformes. |
|
|
|
Fragmentary specimen. |
Member of the Dapediidae.[88] |
||
|
|
GPIH 4864, Hyomandibula |
Member of the Chondrosteidae. This find, which probably originates from the western Baltic basin between Bornholm Island (Denmark) and northeastern Germany, markedly expands the known range of this chondrosteid taxon across the northern part of the strait connecting the Boreal Sea with the Tethys Ocean during the Early Jurassic.[89] The relatively small size of the hyomandibula compared to the largest English material (ca. 40% smaller) may be related to a younger ontogenetic stage of the individual.[89] |
| |
Chondrichthyes
| Genus | Species | Stratigraphic position | Material | Notes | Images |
|---|---|---|---|---|---|
|
|
|
Member of the Hybodontiformes. Related to Hybodus hauffianus and other genera from the southern of Germany |
||
|
|
Teeth |
Member of the Squalomorphii. Small to medium benthonic sharks. |
||
|
|
Teeth |
Member of the Orectolobiformes. Small benthonic sharks. |
||
Sarcopterygii
| Genus | Species | Location | Material | Notes | Images |
|---|---|---|---|---|---|
|
Cf. Coelacanthidae |
|
Scales |
A Coelacanth, member of the family Coelacanthiformes inside Actinistia. The scales recovered are homologous to that found on the Triassic coelacanths Axelia & Wimania. |
||
|
Ptychoceratodus rectangulus |
|
Teeth |
A Lungfish, type member of the family Ptychoceratodontidae inside Dipnoi. A Lungfish of relative small size, related with Freshwater Debris, found associated with Deltaic Deposits. |
||
|
|
|
A Lungfish, member of the Neoceratodontidae inside Ceratodontiformes. A Lungfish, abundant on Europe since the Hettangian to the Bajocian on the Jurassic. The Species are known by a few scarce fragments, and are related with Triassic species found along Germany. |
 Illustration of Ceratodus by Heinrich Harder | |
Reptiles
Ichthyosaurs
| Genus | Species | Stratigraphic position | Material | Notes | Images |
|---|---|---|---|---|---|
|
|
Posterior left half of the cranium. |
A Icthyosaur of the family Stenopterygiidae inside Thunnosauria. A common Toarcian Ichthyosaur, present on multiple layers. The rather exquisite level of preservation has led to know even the coloration. |
 Restoration | |
|
|
Four articulated tail vertebrae. |
An indeterminate ichthyosaur, has been assigned to the species Stenopterygius longifrons |
||
|
|
Partial skull and associated postcranial elements preserved in a concretion |
An indeterminate ichthyosaur. It has an expanded basipterygoid processes on the basisphenoid, only currently known in members of the Ophthalmosauridae |
||
|
|
Presacral centrum, ?sacral centrum |
An indeterminate ichthyosaur. |
||
|
|
Skull, coracoid and associated rib fragment |
An indeterminate ichthyosaur. Assigned to Ichthyosaurus sp., but also suggested affinities to "Leptopterygius" (= Temnodontosaurus) platyodon. |
||
Sauropterygia
| Genus | Species | Stratigraphic position | Material | Notes | Images |
|---|---|---|---|---|---|
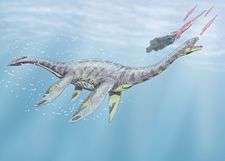
|
|
Three caudal vertebrae, Gastralia, Cervical rib and other postcranial elements |
Non assigned to a concrete genus, probably are related to the Plesiosaurian genera from the Posidonia Shale |
||
|
|
Cervical vertebra, Caudal centrum & Phalanx |
Non assigned to a concrete genus, probably are related to the Plesiosaurian genera from the Posidonia Shale |
||
|
|
Isolated tooth crown & isolated cervical vertebrae |
Non assigned to a concrete genus, probably are related to the Plesiosaurian genus Microcleidus or to Seeleyosaurus |
||
|
|
Three articulated dorsal vertebrae |
Non assigned to a concrete genus, probably are related to the Plesiosaurian genus Microcleidus |
| |
|
|
Incomplete coracoid and associated rib |
Material referred to the family Rhomaleosauridae |
| |
Crocodyliformes
| Genus | Species | Stratigraphic position | Material | Notes | Images |
|---|---|---|---|---|---|
|
|
Cervical vertebra |
Affinities with Goniopholididae inside Neosuchia. Non assigned to a concrete genus, originally labeled as Mesoeucrocodylia indet. can be the earliest representative of the group on Europe. |
||
|
|
Partial rostrum with teeth |
Affinities with Thalattosuchia inside Neosuchia. Probably related to Pelagosaurus |
||
|
|
Incomplete skull and associated osteoderm |
Affinities with Thalattosuchia inside Neosuchia. A marine crocodrylomorph with a diet probably based on fish.[100] It is the best known member of the family Teleosauriade. |
| |
Theropoda
| Genus | Species | Stratigraphic position | Material | Notes | Images |
|---|---|---|---|---|---|
|
Orionides? Indeterminate |
|
Dorsal Vertebrae |
A possible Tetanurae theropod. The affinities of the Specimen aren't clear due to its fragmentary nature. Has been classified as Saurischia indeterminate, although shows clearly characters of the Orionides group (concave articular surfaces and a dished lateral pleurocoel, remnants of the neural arch and postzygapophyses).[101] The vertebrae centrum measures 80 mm, implying a medium-sized theropod (~5 m long).[101] Can be related with Yunyangosaurus. |
||
Sauropoda
| Genus | Species | Stratigraphic position | Material | Notes | Images |
|---|---|---|---|---|---|
|
Gravisauria Indeterminate[103] |
|
|
A Gravisaurian Sauropod. The specimen is believed to be a juvenile, based on the ossification and unfused spine. Has affinities with the genus Tazoudasaurus and it is clearly distinctive form the also toarcian Ohmdenosaurus, who is thought to be more basal.[103] The pelvic girdle elements can be clearly placed among the Sauropoda, on account of the presence of an elongated and strongly dorsally expanded iliac preacetabular process a possible relative.[103] The ischia GG411/3-4 resemble those of Tazoudasaurus in exhibiting a subtriangular iliac peduncle which create a short anteriorly directed expansion to reach the medial acetabular rim, and are slightly less developed than the Genus Barapasaurus.[103] |
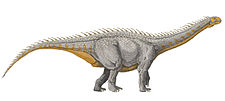 Barapasaurus can be a close relative of the Grimmen Sauropod | |
|
Gravisauria Indeterminate[103] |
|
|
A Gravisaurian Sauropod. The specimen is believed to be a juvenile (its comparatively small size is indicative of belonging to a not fully grown individual) and probably related to the Asian genus Zizhongosaurus, as shares characters with the neural spine V9067.1.[103] GG412 within Gravisauria is indicated by the presence of a well-developed spinodiapophyseal lamina.[103] |
||
Thyreophora
| Genus | Species | Stratigraphic position | Material | Notes | Images |
|---|---|---|---|---|---|
|
|
Right side of the skull, the right lower jaw, caudal vertebrae, neural arches, a radius, a metatarsal, a claw, fragments of ribs, scutes and plates.[104] |
A Thyreophoran ornithischian. Its juvenile status makes controversial its phylogeny.[104] |
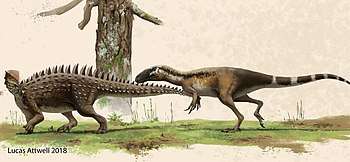 Emausaurus attacked by a theropod | |
Plantae
Megaespores
| Genus | Species | Stratigraphic position | Material | Notes | Images |
|---|---|---|---|---|---|
|
|
|
Affinities with Sphagnopsida inside Sphagnales. Pollen nearly identical to that one found associated with the modern moss genus Sphagnum. Moss related to high humid environments. |
 | |
|
|
|
Affinities with Lycophyta inside Tracheophyta. A relative abundant genus, specially on deltaic deposits. Low herbaceous Lycophyte flora. |
||
|
Acanthotriletes[109] |
|
|
|
Affinities with Lycophyta inside Tracheophyta. This Spores, rather abundant are correlated with humid settings, and come from mostly herbaceous-arbustive plants. |
|
|
Hughesisporites[109] |
|
|
|
Affinities with Lycophyta inside Tracheophyta. Spores from low herbaceous flora, linked mostly to humid environments with abundant freshwater. |
|
|
|
|
Affinities with Lycophyta inside Tracheophyta. Low herbaceous flora from humid envrironments |
||
|
|
|
Affinities with Lycophyta inside Tracheophyta. Abundant on humid strata, mostly related with deltaic facies. |
||
|
|
|
Affinities with Lycophyta inside Tracheophyta. Rather rare than other similar genera, found mostly on deltaic facies. |
||
|
Trachytriletes[109] |
|
|
|
Affinities with Lycopodiaceae inside Lycopodiopsida. Represents herbaceous Lycophytes of small to medium size (10–40 cm), that are found mostly on deltaic deposits. |
|
|
Lycopodiumsporites[109] |
|
|
|
Affinities with Lycopodiaceae inside Lycopodiopsida. Resemble spores of the modern genus Lycopodium. If it belongs to a similar genus, represent low herbaceous flora spores. |
 |
|
Reticulatisporites[109] |
|
|
|
Affinities with Lycopodiaceae inside Lycopodiopsida. Resembles the modern genera Lycopodiella and Huperzia, characteristic small to medium (20–90 cm) herbaceous Lycopites, found on environments with abundant water. |
|
|
|
|
Affinities with the Lycopodiopsida inside Lycophyta. Lycophyte spores of rather uncertain affinities, are more common on fluvial deposits. |
||
|
Paxillitriletes[113] |
|
|
|
Affinities with the Isoetales inside Lycophyta. Spores related with modern Isoetes, representing Small plants related with water bodies.[114] It comprises the main Megaspore zonation of the Toarcian of Poland, being the most abundant spore found. |
 |
|
Minerisporites[113] |
|
|
|
Affinities with Isoetaceae inside Lycopsida. Related to plant similar to Isoetes lacustris, present on flooded basins, and other ecosystems with relative abundant water supply.[115] |
|
|
|
|
Affinities with Isoetaceae inside Lycopsida. |
||
|
|
|
Affinities with the genus Selaginella inside Selaginellaceae. Herbaceous Moss.[117] Relatively abundant Pollen Genera |
 | |
|
Horstisporites[107] |
|
|
|
Affinities with the Selaginellaceae inside Lycopsida. Less abundant than othe Lycophite Spores, its abundance increases near Deltaic deposits. |
|
|
|
|
Affinities with the Selaginellaceae inside Lycopsida. Among the most abundant genera of Pollen present on the Formation and the south of Fennoscandia |
||
|
Valvisisporites[110] |
|
|
|
Affinities with the Selaginellaceae inside Lycopsida. This herbaceous Lycophyte flora appears more on the southern boreholes, linked to large wood debris. |
|
|
Maexisporites[110] |
|
|
|
Affinities with the Selaginellaceae inside Lycopsida. Herbaceous flora spores linked to deltaic facies. |
|
|
Striatriletes[110] |
|
|
|
Affinities with the Selaginellaceae inside Lycopsida. |
|
|
Foveosporites[109] |
|
|
|
Affinities with the Selaginellaceae inside Lycopsida. Less abundant than other similar spores, found on a few samples on all the boreholes. |
|
|
Klukisporites[109] |
|
|
|
Affinities with the Selaginellaceae inside Lycopsida. Represents large spores from herbaceous lycophytes, that are more abundant on the south of the Toarcian polish basin than on the north. |
|
|
|
|
Affinities with Pleuromeiales inside Lycophyta. The Plueromeiales where tall Lycophites (2 to 6 m) common on the Trassic. Probably come from a relict genus. |
||
|
|
|
Affinities with the Cynepteridaceae inside Schizaeaceae. Related to Ferns similar to Cynepteris, from the late triassic of North America. |
||
|
Reticulatisporites[106] |
|
|
|
Affinities with Lygodiaceae inside Schizaeales. Spores nearly identical to that one found associated with the modern moss genus Lygodium. Arboreal moss related to high humid environments, being major fuel for peat fires. |
 |
|
Perinopollenites[109] |
|
|
|
Affinities with Gymnospermopsida inside Tracheophyta. Pollen that resemble those of paleozoic genera such as Gangamopteris, with arboreal built. |
|
|
Chasmatosporites[109] |
|
|
|
Affinities with Gymnospermopsida inside Tracheophyta. Pollen that resemble those of paleozoic genera such as Gangamopteris, with arboreal built. |
|
|
|
|
Affinities with the Filicopsida inside Monilophyta. Fern spores of uncertain placement |
||
|
|
|
Affinities with the Polypodiidae inside Filicopsida. Fern spores of uncertain placement. |
||
|
|
|
Affinities with the Polypodiidae inside Filicopsida. Fern spores of uncertain placement |
||
|
Osmundacidites[6] |
|
|
Spores |
Affinities with Osmundaceae inside Pteridophyta. Spores nearly identical to that one found associated with the modern fern genus Osmunda. Members of the genus Osmunda have been found on coeval age strata on Sweden. |
|
|
|
|
Affinities with the Marsileaceae inside Salviniales. Represents spore from fully acuatic ferns, found associated with fluvial or deltaic deposits, where probably formed large underwater colonies. |
 | |
|
|
|
Affinities with the Gleicheniaceae inside Polypodiidae. Resemble the modern Gleichenia Spores, and proably represent a similar genus or a member of it. Fern related to large colonies, found mostly on humid environments. |
 | |
|
|
|
Affinities with the Marattiaceae inside Polypodiidae. Resemble the modern Marattia spores, probably belonging to a similar genus, related with large sized herbaceous ferns of humid environments. |
 | |
|
Camptotriletes[109] |
|
|
|
Affinities with the Marattiaceae inside Polypodiidae. |
|
|
|
|
Affinities with the Matoniaceae inside Polypodiidae. The so-called comb-ferns, found forming large colonies on humid settings. |
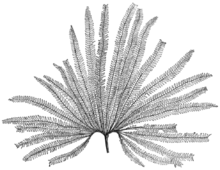 | |
|
|
Spores |
Affinities with Cyatheaceae inside Cyatheales. Cyathidites minor almost certainly belong to well known Mesozoic species Coniopteris hymenophylloides and to other fossil cyatheaceous or dicksoniaceous ferns such as Eboracia lobifolia and Dicksonia mariopteri. |
||
|
|
|
Affinities with the Gymnospermophyta inside Spermatophyta. Pollen from uncertain Gimnosperm affinities. |
||
|
|
|
Affinities with the Cycadales inside Cycadopsida. Originally was trought to come from Angiosperms. |
||
|
Psophosphaera[109] |
|
|
|
Affinities with the Cycadales inside Cycadopsida. Cycad Pollen, coming from arbustive to low arboreal flora. |
|
|
|
Pollen |
Named originally Pollenites apertus, they resemble pollen grains of the genus Cycas. Later works refer them to modern pollen grains that proper of wrote that resemble ?Cycadopsida (?Cycadales), concretely the genus Encephalartos. Alternatively, can be Pollen from members of Ginkgopsida (?Gnetales).[119] |
 | |
|
|
|
Affinities with the inside Cycadopsida.[120] Very abundant Pollen. |
||
|
Pseudowalchia[109] |
|
|
|
Affinities with Voltziales inside Coniferae. Primitive Conifer and possible relict taxon |
|
|
|
|
Affinities with the Pinidae inside Coniferae. |
||
|
Rotundipollenites[109] |
|
|
|
Affinities with Pinaceae inside Coniferae. |
|
|
|
|
Affinities with Pinaceae inside Coniferae. Resemble modern Pinus Pollen, probably belonging to a similar Genus. |
 | |
|
Quadraeculina[106] |
|
|
|
Affinities with Pinaceae inside Coniferae. Pollen From arbustive to arboreal plants, resembling the modern genus Picea |
 |
|
|
Pollen |
Affinities with Abietoideae inside Coniferae. Pollen From arbustive to arboreal plants, resembling the pollen of the modern genus Tsuga |
| |
|
|
Pollen |
Affinities with Sciadopityaceae inside Coniferae. This pollen resembles the present on the modern Cupressaceae Sciadopitys |
 | |
|
|
|
Affinities with Podocarpaceae inside Pinopsida. Pollen From arbustive to arboreal plants |
||
|
|
Pollen |
Affinities with Podocarpaceae inside Pinopsida. Pollen From arbustive to arboreal plants |
||
|
|
|
Affinities with the Cheirolepidiaceae inside Coniferae. Is very abundant on hot and dry settings, found specially on the Brody-Lubienia Borehole. |
||
|
|
|
Affinities with the Cupressaceae inside Coniferae. The Pollen from this genus is similar to the present on the modern Fitzroya. |
 | |
|
|
|
Affinities with the Araucariaceae inside Coniferae. Resemble the pollen from the modern genus Agathis. |
| |
|
|
Pollen |
Affinities with Araucariaceae inside Pinopsida. Pollen From arbustive to arboreal plants |
||
|
Tricolpites[116] |
|
|
|
Affinities with Angiospermae and probably Magnoliophyta. Possible pollen from primigenial Flowering plants or relatives |
|
|
Clavatipollenites[109] |
|
|
|
Affinities with Angiospermae and probably Magnoliophyta. Possible pollen from primigenial Flowering plants or relatives |
|
Megaflora
The Lublin Upland fluvial sandstones contain diverse types of fossil flora, associated genera and species only with Lower Jurassic sediments. Originally, while studying the Carboniferous flora from the boreholes in the area of the planned "Bogdanka" mine, appeared typical flora in similar to Jurassic formations.[121] The age of the plant material was not determined concretely until 2020, where was recovered as being Lower Toarcian in age (with some parts probably recovering Latest Pliensbachian strata).[122] Lublin lias is dominated by cycads and Bennetites Ginkgoales and pinnate, ferns occur sporadically, all on a conglomerate with numerous species occurs in the bottom of the Toarcian, where the deposits are filled with of coal, mudstone, sandstone and clay siderite (reworked from the Carboniferous), as well as pebbles from Devonian limestones.[123] Due to the fact that similar Boreholes and deposits nearby show similar characters it should be assumed that we have to dealing with the end of a river, that eroded the nearest Devonian-Carboniferous deposits (At the northeast), being brought by flowing waters and deposited in the aquatic environment-inland.[123] Vegetation mostly grew outside the sedimentation area, as well as on shores and shallows.[124]
| Genus | Species | Stratigraphic position | Material | Notes | Images |
|---|---|---|---|---|---|
|
|
Stems |
Affinities with the Equisetales inside Equisetopsida. Very rare. Local Equisetales obtained a considerable size, comparable to modern subtropical bamboos, close to lakes and in the wettest environments |
||
|
|
Pinnae |
Affinities with the Peltaspermales inside Pteridospermatophyta. Bipinnate leaves, rachis longitudinally striated, with a long petiole and secondary rachises inserted oppositely to suboppositely on the upper side of the primary rachis. This Leaves belong to large Aboreal Ferns related with dry environments. |
||
|
|
Pinnae |
Affinities with the Umkomasiaceae inside Pteridospermatophyta. Is very similar to Rhaphidopteris, characterized by usually bi-tripinnate, coriaceous leaves with narrow segments. It belongs to Large (up to 25 m tall) arboreal Fern-like plants. |
||
|
|
Ovulate Structure |
Affinities with the Caytoniaceae inside Caytoniales. Represents the Ovulate organs or large Tree ferns, and is related with the middle Jurassic flora of United Kingdom. |
 | |
|
|
Pinnae |
Affinities with the Caytoniaceae inside Caytoniales. Sagenopteris is most likely the Pinnae of the Tree Fern that also contain Caytonia. |
 | |
|
|
Pinnae |
Affinities with the Bennettitales inside Bennettitopsida. Anomozamites is characterised by slender, (almost) completely and regularly segmented leaves whose leaflets are generally as long as broad or, at maximum, two times as long as broad. This genus is related with more arboreal Bennetitalean flora. Shows coriaceous leaves and is a genus linked more with dry climates. |
 | |
|
|
Pinnae |
Affinities with the Bennettitales inside Bennettitopsida. A more arbustive type of Bennetite, abundant on the Pliensbachian-Toarcian strata along Eurasia, related to rather dry climates. |
.jpg) | |
|
|
Pinnae |
Affinities with the Bennettitales inside Bennettitopsida. This Bennetite has a leaf similar to Nilssonia, large, lanceolate in outline with coriaceous structure, like the modern angiosperm Philodendron. Is related with rather Dry-hot environments, with less Fern flora. |
||
|
|
Pinnae |
Affinities with the Cycadeoidaceae inside Bennettitales. The most abundant plant macrofossil locally, and the most diverse found on the Lublin coals. It represents a Cycad Like plant with Coriaceous leaves. Is related by some botanists with the Bennetite branch that led to Angiosperms. |
| |
|
|
Bennetite "Flower" |
Affinities with the Cycadeoidaceae inside Bennettitales. The Bennetite flowers are the main organ that links this relatives with the Cycas with modern Angiosperms. Probably the Pollen assigned to Angiosperms come from plants with this "Flowers". |
||
|
|
Pinnae |
Affinities with the Williamsoniaceae inside Bennettitopsida. Leaves from Arboreal Bennetites, similar to the modern Cyca Encephalartos woodii, with robust Trunks, built for Dry and hot climates. |
 | |
|
|
Leaves |
Affinities with the Ginkgoaceae inside Ginkgoidae. Linked to the Hettangian-Sinemurian flora from Greenland and Skane, but also with coeval flora from the Sorthat Formation. Is the main Tree flora recovered locally. |
 | |
|
|
Leaves |
Affinities with the Cheirolepidiaceae inside Pinales. The type Leave from the Cheirolepidaceae family (as Cheirolepis, the genus that give name to the family, is a junior synonym), appears to be linked with Hot climates, able to survive in dry, extreme conditions, and been fire tolerant. |
||
Footnotes
- Barth, G., Pieńkowski, G., Zimmermann, J., Franz, M., & Kuhlmann, G. (2018). Palaeogeographical evolution of the Lower Jurassic: high-resolution biostratigraphy and sequence stratigraphy in the Central European Basin. Geological Society, London, Special Publications, 469(1), 341-369.
- Kopik J. and Marcinkiewicz T. 1997. Jura dolna: Biostratygrafia. In: S. Marek and M. Pajchlowa (Eds), The epicontinental Permian and Mesozoic in Poland (in Polish with English summary). Prace Paƒstwowego Instytutu Geologicznego, 153: 196-205.
- Kopik, J. (1998). Lower and Middle Jurassic of the north-eastern margin of the Upper Silesian Coal Basin. Biuletyn Państwowego Instytutu Geologicznego, 378, 67-129.
- Barski, M., & Leonowicz, P. (2002). Dinoflagellates of Lower Jurassic outcrops at Kozłowice and Boroszów (southern Poland). Przegląd Geologiczny, 50(5), 411-414.
- Pieńkowski, G., Hodbod, M., & Ullmann, C. V. (2016). Fungal decomposition of terrestrial organic matter accelerated Early Jurassic climate warming. Scientific reports, 6, 31930.
- Rogalska,M. (1954) Spore and pollen analysis of the Liassic Coal of Blanowice in Upper Silesia [ Analyza sporowo-pylkowa Liasowego Wegla Blano-Wickiego z Gornego Slaska ] Biuletyn - Instytutu Geologiczny Vol. 89 P. 1- 46
- Leonowicz, P. (2008). Trace fossils from the Lower Jurassic Ciechocinek Formation, SW Poland. Volumina Jurassica, 6(6), 89-98.
- Keighley, D. G., & Pickerill, R. K. (1995). Commentary: the ichnotaxa Palaeophycus and Planolites: historical perspectives and recommendations.
- Marcinkiewicz,T. (1971) The stratigraphy of the Rhaetian and Liasin Poland based on megaspore investigations [ Stratygrafia Retyku i Liasu w Polsce na Podstawie badan megasporowych. ] Prace Instytut Geologiczny,(Warsaw) Vol. 65 P. 1- 57
- Denckmann, A. (1887). Ueber die geognostischen Verhältnisse der Umgegend von Dörnten nördlich Goslar: mit besonderer Berücksichtigung der Fauna des oberen Lias (Vol. 8, No. 2). In Commission bei der S. Schropp'schen Hof-Landkartenhandlung (JH Neumann).
- ZESSIN, W. (2010). Der Dobbertiner Jura (Lias ε, Mecklenburg) und seine Bedeutung für die Paläoentomologie. Virgo, Mitteilungsblatt des Entomologischen Vereins Mecklenburg, 13(1), 4-9.
- Lehmann, U. (1967). Ammoniten mit Kieferapparat und Radula aus Lias-Geschieben. Paläontologische Zeitschrift, 41(1-2), 38-45.
- Howarth, M. K., & Rawson, P. F. (1965).The Liassic succession in a clay pit at Kirton in Lindsey, north Lincolnshire. Geological Magazine, 102(3), 261-266.
- van de Schootbrugge, B., McArthur, J. M., Bailey, T. R., Rosenthal, Y., Wright, J. D., & Miller, K. G. (2005). Toarcian oceanic anoxic event: An assessment of global causes using belemnite C isotope records. Paleoceanography, 20(3).
- Ansorge, J. & Grimmebergen, G. (2016):Grätensandsteine und andere Geschiebe des oberen Lias (Toarcium) aus Norddeutschland [Upper Liassic sandstones with fish remains (so-called Grätensandsteine) and other Toarcian glacial erratics from northern Germany]. Geschiebekunde aktuell 32 (4): 121-141, 12 Abb
- Lehmann, U. (1966). Dimorphismus bei Ammoniten der Ahrensburger Lias-Geschiebe. Paläontologische Zeitschrift, 40(1-2), 26-55.
- Hoffmann, R., Schultz, J. A., Schellhorn, R., Rybacki, E., Keupp, H., Lemanis, R., & Zachow, S. (2014). Non-invasive imaging methods applied to neo-and paleo-ontological cephalopod research.
- LürnG, G. (1995). Geschiebezählungen-eine terminologische Richtigstellung. Geschiebekunde aktuell, 11(4), 109-112.
- Hoffmann, K., & Martin, G. P. (1960). Die Zone desDactylioceras tenuicostatum (Toarcien, Lias) in NW-und SW-Deutschland. Paläontologische Zeitschrift, 34(2), 103-149.
- Schlögl, J., Košt’ák, M., & Hyžný, M. (2012). First record of a gladius-bearing coleoid Teudopsis bollensis Voltz (Cephalopoda, Coleoidea) in the Toarcian of the Western Carpathians (Slovakia). Paläontologische Zeitschrift, 86(4), 367-375.
- Riegraf, W. (1997). On the proposed conservation of the names Geopeltis Regteren Altena, 1949, Geoteuthis Muenster, 1843, Jeletzkyteuthis Doyle, 1990, Loligosepia Quenstedt, 1839, Parabelopeltis Naef, 1921, Paraplesioteuthis Naef, 1921 and Belemnotheutis montefiorei Buckman, 1880 (Mollusca, Coleoidea). BULLETIN OF ZOOLOGICAL NOMENCLATURE, 54, 184-184.
- Fuchs, D., & Weis, R. (2008). Taxonomy, morphology and phylogeny of Lower Jurassic loligosepiid coleoids (Cephalopoda). Neues Jahrbuch Für Geologie Und Paläontologie - Abhandlungen, 249(1), 93–112. doi:10.1127/0077-7749/2008/0249-0093
- Kutscher, M. (1988). Zur Invertebratenfauna und Stratigraphie des oberen Pliensbachien von Grimmen (DDR), Echinodermata. Freiberger Forschungshefte C, 419, 62-70.
- Geinitz, F. E. (1884). Ueber die Fauna des Dobbertiner Lias. Zeitschrift der deutschen geologischen Gesellschaft, 566-583.
- Ansorge, J. (2007). Lower Jurassic clay pit of Klein Lehmhagen near Grimmen. The Central European Basin System–from the Bottom to the Top. Geo-Pomerania, Szczechin, 37-41.
- Leonowicz, P. M. (2016). Tubular tempestites from Jurassic mudstones of southern Poland. Geological Quarterly, 60(2), 385-394.
- E. Herrig Zur Taxonomie und Evolution der Gattung Kinkelinella Martin, 1960 (Ostracoda) im Unteren Jura von Mittel- und Nordwesteuropa Zeitschrift für Geologische Wissenschaften, Berlin, 13 (6) (1985), pp. 715-723
- E. Herrig Die polycopiden Ostrakoden aus dem thüringischen Lias Zeitschrift für Geologische Wissenschaften, Berlin, 9 (6) (1981), pp. 675-696
- T. Pietrzenuk Zur Mikrofauna einiger Lias vorkommen in der Deutchen Demokratischen Republik Freiberger Forschunghelfe, H(C), 113 (1961), pp. 1-129
- E. Dreyer Mikrofossilem des Rät und Lias von SW-Brandenburg Geologische Jahrbuch, Hannover, 1 (1967), pp. 491-531
- E. Herrig Die Gattung Ogmoconchella—Arten (Ostracoda) im Lias von Thüringen Zeitschrift für Geologische Wissenschaften, Berlin, 9 (5) (1981), pp. 561-579
- E. Herrig Die Gattung Ogmoconcha Triebel, 1941 (Ostracoda) im Lias von Thüringen Zeitschrift für Geologische Wissenschaften, Berlin, 9 (2) (1981), pp. 207-219
- E. Herrig Ostrakoden der Gattungen Ledahia and Pseudohealdia (Familie: Healdiidae Harlton) aus dem Lias von Thüringen Zeitschrift für Geologische Wissenschaften, Berlin, 8 (12) (1980), pp. 1539-1551
- E. Herrig Ostracoden aus dem Ober-Domèrien von Grimmen westlich Griefswald (Teil I) Geologie, Berlin, 18 (1969), pp. 446-471
- E. Herrig Ostracoden aus dem Ober-Domèrien von Grimmen westlich Griefswald (Teil II) Geologie, Berlin, 18 (1969), pp. 1072-1101
- Close E. Herrig, H. Richter Zur Entwicklung der Cytheropterinae (Ostracoda Crustacea) im oberen Lias von Mitteleuropa Neues Jahrbuch für Geologie und Paläontologie Abhandlungen, Stuttgart, 180 (2) (1990), pp. 239-257
- E. Herrig Ostrakoden aus dem Lias von Thüringen. Die Familien Progonocytheridae, Cytherettidae und Brachycytheridae Zeitschrift für Geologische Wissenschaften, 10 (1982), pp. 1449-1461
- E. Herrig Ostracoden aus dem Lias von Thüringen. Die Familien Trachyleberididae, Paradoxostomatidae, Cytherellidae sowie Nachtrag zu den Paracyprididae Zeitschrift für Geologische Wissenschaften, 10 (1982), pp. 231-243
- E. Herrig Die Gattung Bairdia (Ostracoda, Crustacea) im Lias von Thüringen Zeitschrift für Geologische Wissenschaften, Berlin, 7 (5) (1979), pp. 641-661
- E. Herrig Ostrakoden aus dem Lias von Thüringen. Die Gattungen Bairdia (Teil II), Fabalacypris and Bairdiacypris Zeitschrift für Geologische Wissenschaften, Berlin, 7 (6) (1979), pp. 763-782
- Selden, Paul A.; Dunlop, Jason A. (2014). "The first fossil spider (Araneae: Palpimanoidea) from the Lower Jurassic (Grimmen, Germany)". Zootaxa. 3894 (1): 161–168. doi:10.11646/zootaxa.3894.1.13.
- Ansorge, J. (2003). Insects from the lower Toarcian of middle Europe and England. Acta zoologica cracoviensia, 46(SUPPL.), 291-310.
- Q. Q. Zhang, W. Mey, J. Ansorge, T. A. Starkey, L. T. McDonald, M. E. McNamara, E. A. Jarzembowski, W. Wichard, R. Kelly, X. Y. Ren, J. Chen, H. C. Zhang, and B. Wang. 2018. Fossil scales illuminate the early evolution of lepidopterans and structural colors. Science Advances 4:e1700988
- A. Handlirsch. 1906. Die Fossilen Insekten und die Phylogenie der Rezenten Formen, parts I-IV. Ein Handbuch fur Palaontologen und Zoologen 1-640
- W. Zessin. 1983. Revision der mesozoischen Familie Locustopsidae unter Berücksichtigung neuer Funde (Orthopteroida, Caelifera). Deutsche Entomologische Zeitschrift 30:173-237
- W. Zessin. 1987. Variabilität, Merkmalswandel und Phylogenie der Elcanidae im Jungpaläozoikum und Mesozoikum und die Phylogenie der Ensifera. Deutsche Entomologische Zeitschrift 34(1-3):1-76
- A. Handlirsch. 1939. Neue Untersuchungen über die fossilen Insekten mit Ergänzungen und Nachträgen sowie Ausblicken auf phylogenetische, palaeogeographische und allgemein biologische Probleme. II Teil. Annalen des Naturhistorischen Museums in Wien 49:1-240 [M. Clapham/J. Karr/M. Clapham]
- W. Zessin. 1987. Variabilität, Merkmalswandel und Phylogenie der Elcanidae im Jungpaläozoikum und Mesozoikum und die Phylogenie der Ensifera. Deutsche Entomologische Zeitschrift 34(1-3):1-76
- W. Zessin. 1988. Neue Saltatoria (Insecta) aus dem Oberlias Mitteleuropas. Freiberger Forschungshefte C 419:107-121
- J. Ansorge. 1996. Insekten aus dem oberen Lias von Grimmen (Vorpommern, Norddeutschland). Neue Paläontologische Abhandlungen 2:1-132
- J. Ansorge. 1999. Aenne liasina gen. et sp. n. - the most primitive non biting midge (Diptera: Chironomidae: Aenninae subfam. n.) - from the Lower Jurassic of Germany. Polskie Pismo Entomologiczne 68:431-443
- Ansorge, J. (1993). Dobbertiniopteryx capniomimus gen. et sp. nov. die erste Steinfliege (Insecta: Plecoptera) aus dem europäischen Jura. Paläontologische Zeitschrift, 67(3-4), 287-292.
- W. Krzemiński and W. Zessin. 1990. The Lower Jurassic Limoniidae from Grimmen (GDR) (Dipt. Nematocera). Deutsche Entomologische Zeitschrift 37:39-43
- Kopeć, K., Ansorge, J., Soszyńska-Maj, A., & Krzemiński, W. (2018). Revision of the genus Mesotipula Handlirsch, 1920 (Diptera, Limoniidae, Architipulinae) from the Lower Jurassic of Northeast Germany. Historical Biology, 1-8.
- KRZEMIŃSKI, W., & ANSORGE, J. A new rhagionid fly from the Lower. Jurassic. Polskie Pismo Entomologiczne, 74, 3.
- A. Handlirsch. 1920. Palaeontologie. Handbuch der Entomologie 3:117-208
- N. S. Kalugina. 1985. Infraorders Psychodomorpha, Tipulomorpha and Culicomorpha. Dvukrylye nasekomye Yury Sibiri 33-113
- J. Ansorge. 1994. Tanyderidae and Psychodidae (Insecta: Diptera) from the Lower Jurassic of northeastern Germany. Palaeontologische Zeitschrift 68:199-210
- ANSORGE, J. (2001). Lower Jurassic Hennigmatidae (Diptera) from Germany. Studia dipterologica, 8(1), 97-102.
- Ansorge, J. (1993). Parabittacus analis Handlirsch 1939 und Parabittacus lingula (Bode 1953), Neorthophlebiiden (Insecta: Mecoptera) aus dem Oberen Lias von Deutschland. Paläontologische Zeitschrift, 67(3-4), 293-298.
- A. Wendt. 1940. Liasocoris hainmülleri n. sp., eine fossile Wanze aus Mecklenburg. Archiv des Vereins der Freunde der Naturgeschichte in Mecklenburg, Neue Folge 15:18-20
- G. Enderlein. 1909. Zur Kenntnis frühjurassischer Copeognathen und Coniopterygiden und über das Schicksal der Archipsyiliden. Zoologischer Anzeiger 34:770-776
- A. Handlirsch. 1921. Palaeontologie. Handbuch der Entomologie 3:209-304
- F. E. Geinitz. 1880. Der Jura von Dobbertin in Mecklenburg und seine Versteinerungen. Zeitschrift der Deutschen Geologischen Gesellschaft 32:510-535
- D. E. Shcherbakov. 2012. More on Mesozoic Membracoidea (Homoptera). Russian Entomological Journal 21:15-22
- A. P. Rasnitsyn, J. Ansorge, and H. C. Zhang. 2006. Ancestry of the orussoid wasps, with description of three new genera and species of Karatavitidae (Hymenoptera = Vespida: Karatavitoidea stat. nov.). Insect Systematics & Evolution 37:179-190
- Ansorge, J., & Schlüter, T. (1990). The earliest chrysopid: Liassochrysa stigmatica ng, n. sp. from the Lower Jurassic of Dobbertin, Germany. Neuroptera International, 6(2), 87-93.
- Krüger, L. (1922). Hemerobiidae. Beiträge zu einer Monographie der Neuropteren-Familie der Hemerobiiden. Stettiner Entomologische Zeitung, 83, 138-172.
- A. Bode. 1953. Die Insektenfauna des Ostniedersachsischen Oberen Lias. Palaeontographica Abteilung A 103:1-375
- W. Zessin. 1982. Durchsicht einiger liassischer Odonatopteroida unter Berücksichtigung neuer Funde von Dobbertin in Mecklenburg. Deutsche Entomologische Zeitschrift 29:101-106
- Zessin, W., & Ansorge, J. (1987). Magnasupplephlebia intercalaria n. sp.‐eine neue Anisozygopterenart aus dem oberen Lias von Mitteleuropa.(Insecta, Odonata). Deutsche Entomologische Zeitschrift, 34(4‐5), 383-386.
- J. Ansorge. 2004. Insekten aus Liasgeoden der Ahrensburger Geschiebesippe - mit einem Ausblick auf lokale Anreicherungen von Liäsgeoden in Mecklenburg-Vorpommern. Archiv für Geschiebekunde 3:779-784
- J. Cowley. 1942. Descriptions of some genera of fossil Odonata. Proceedings of the Royal Entomological Society of London. Series B, Taxonomy 11:63-78
- W. Zessin. 1981. Ein Hymenopterenflügel aus dem oberen Lias bei Dobbertin, Bezirk Schwerin. Zeitschrift für Geologische Wissenschaften 9:713-717
- A. P. Rasnitsyn, J. Ansorge, and W. Zessin. 2003. New hymenopterous insects (Insecta: Hymenoptera) from the lower Toarcian (Lower Jurassic) of Germany. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen 227:321-342
- P. Vršanský and J. Ansorge. 2007. Lower Jurassic cockroaches (Insecta: Blattaria ) from Germany and England. African Invertebrates 48(1):103-126
- F. E. Geinitz. 1883. Die Flötzformationen Mecklenburgs. Archiv des Vereins der Freunde der Naturgeschichte in Mecklenburg 37:1-151
- S. H. Scudder. 1886. A review of Mesozoic cockroaches. Memoirs of the Boston Society of Natural History 3:439-484
- E. Geinitz. 1894. Die Kanferreste des Dobbertiner Lias. Archiv des Vereins der Freunde der Naturgeschichte in Mecklenburg 48:71-78
- E. E. Maxwell and S. Stumpf. 2017. Revision of Saurorhynchus (Actinopterygii: Saurichthyidae) from the Early Jurassic of England and Germany. European Journal of Taxonomy 321:1-29
- Konwert, M., & Hörnig, M. (2018). Grimmenichthys ansorgei, gen. et sp. nov. (Teleostei, “Pholidophoriformes”), and other “pholidophoriform” fishes from the early Toarcian of Grimmen (Mecklenburg-Western Pomerania, Germany). Journal of Vertebrate Paleontology, 38(3), e1451871. doi:10.1080/02724634.2018.1451871
- Lehmann, U. (1971). Faziesanalyse der Ahrensburger Liasknollen auf Grund ihrer Wirbeltierreste. Mitteilungen aus dem Geologischen Institut der Technischen Universität Hannover, 10, 21-42.
- Konwert, M., and S. Stumpf. 2017. Exceptionally preserved Leptolepidae (Actinopterygii, Teleostei) from the late Early Jurassic FossilLagerst€atten of Grimmen and Dobbertin (Mecklenburg-Western Pomerania, Germany). Zootaxa 4243:249–296
- Agassiz, L. (1832) Untersuchungen über die fossilen Fische der Lias-Formation—Aus einem Briefe des Vfs. an Professor Bronn. Jahrbuch für Mineralogie, Geognosie, Geologie und Petrefaktenkunde, 3, 139–149.
- Thies, D. 1989. Sinneslinien bei dem Knochenfisch Lepidotes elvensis (Blainville 1818) (Actinopterygii, Semionotiformes) aus dem Oberlias (Unter-Toarcium) von Grimmen in der DDR. Neues Jahrbuch fur Geologie und Pal € €aontologie, Monatshefte 1989:692–704.
- S. Stumpf, J. Ansorge, C. Pfaff and J. Kriwet. 2017. Early Jurassic diversification of pycnodontiform fishes (Actinopterygii, Neopterygii) after the end-Triassic extinction event: evidence from a new genus and species, Grimmenodon aureum. Journal of Vertebrate Paleontology 37:e1344679
- Thies, D. (1988). Dapedium pholidotum (AGASSIZ, 1832) - (Pisces, Actinopterygii) from Unter-Toarcium NW-Germany. Geologica et Palaeontologica , (22), 89-121.
- Stumpf, S. (2017). A Synoptic Review of the Vertebrate Fauna from the "Green Series" (Toarcian) of Northeastern Germany with Descriptions of New Taxa: A Contribution to the Knowledge of Early Jurassic Vertebrate Palaeobiodiversity Patterns (Doctoral dissertation, Mathematisch-Naturwissenschaftliche Fakultät der Ernst-Moritz-Arndt-Universität Greifswald).
- J. J. Hornung and S. Sachs. 2020. First record of Gyrosteus mirabilis (Actinopterygii, Chondrosteidae) from the Toarcian (Lower Jurassic) of the Baltic region. PeerJ 8:e8400
- M. W. Maisch and J. Ansorge. 2004. The Liassic ichthyosaur Stenopterygius cf. quadriscissus from the Lower Toarcian of Dobbertin (northeastern Germany) and some considerations on Lower Toarcian marine reptile palaeobiogeography. Palaeontologische Zeitschrift 78(1):161-171
- GEINITZ, F.E. 1900a. Ichthyosaurus von Dobbertin.- Archiv des Vereins der Freunde der Naturgeschichte in Mecklenburg 54: 382-383.
- ZESSIN, W. 1995. Saurierfund im Lias epsilon von Grimmen, Kreis Nordvorpommern (Fundbericht). - Geschiebkunde Aktuell 11 (4): 113.
- ZESSIN, W. 1998.20 Jahre geowissenschaftliche Freizeitforschung in Westmecklenburg.- Geschiebekunde Aktuel114 (1): 1-10
- ZESS1N, W. 2001. Ichthyo-Saurierfunde und KrokodilschSxtel aus dem Lias von Klein Lehmhagen bei Grimmen, Kreis Nordvorpommern. - NABU Nachrichten Mecklenburg-Vorpommern 2001 (1): 7-9.
- LEHMANN, U. 1968. Stratigraphie und Ammonitenfiihrung der Ahrensburger Glazial-Geschiebe aus dem Lias epsilon (= Unt. Toarcium). - Mitteilung des Geologischen Staatsinstituts Hamburg 37: 41-68.
- LIERL, H.-J. 1990. Die Ahrensburger Geschiebesippe. - Fossilien 1990 (6): 256-267.
- MOTHS, H. 1994. Ein seltenes Ichthyosaurus-Gebil3 als Geschiebe aus Mecklenburg. - Der Geschiebesammler 27 (1): 15-22.
- Sachs, S., Hornung, J. J., Lierl, H.-J., & Kear, B. P. (2016). Plesiosaurian fossils from Baltic glacial erratics: evidence of Early Jurassic marine amniotes from the southwestern margin of Fennoscandia. Geological Society, London, Special Publications, 434(1), 149–163. doi:10.1144/sp434.14
- STUMPF, S. 2016. New information on the marine reptile fauna from the lower Toarcian (Early Jurassic) “Green Series” of North-Eastern Germany. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen 280(1):87–105.
- Mueller-Töwe, I. J. (2006). Feeding options in Steneosaurus bollensis (Mesoeucrocodylia. Thalattosuchia). Hantkeniana Spec, 5, 46-48.
- F. v. Huene. 1966. Ein Megalosauriden-Wirbel des Lias aus norddeutschem Geschiebe [A megalosaurid vertebra from the Lias of a north German boulder]. Neues Jahrbuch für Geologie und Paläontologie 1966(5):318-319
- Stumpf, S., & Meng, S. (2013). Dinosaurier aus Nordostdeutschland: Verschleppt. Biologie in unserer Zeit, 43(6), 362-368.
- S. Stumpf, J. Ansorge, and W. Krempien. 2015. Gravisaurian sauropod remains from the marine late Early Jurassic (Lower Toarcian) of North-Eastern Germany. Geobios 48:271-279
- Haubold, H. 1990. Ein neuer Dinosaurier (Ornithischia, Thyreophora) aus dem Unteren Jura des nördlichen Mitteleuropa. Revue de Paleobiologie 9(1):149-177. [In German]
- Haubold, H. (1991). Der Greifswalder Dinosaurier ‘‘Emausaurus’’. Fundgrube, 27(2), 51-60.
- DOMAGAŁA M. & KOŁCON I. 1983. Zbiorowiska roślinności węglotwórczej liasowego węgla brunatnegoz Poręby koło Zawiercia (summary: Vegetation assemblages forming Lias brown coals in the Poręba area near Zawiercie). Kwart. Geol., 27(3): 503–516.
- Marcinkiewicz, T. (1973). Otozamites falsus (Bennettitales) from the Upper Liassic of the Holy Cross Mts, Poland. Acta Palaeontologica Polonica, 18(2).
- Dadlez, R., Kopik, J., Marcinkiewicz, T., & Szymborsky, L. (1964). Results obtained in Bore-Hole Mechowo IG 1. Inst. Geol. Bulletin, 189, 1-156.
- Rogalska,M. (1971) Division of the Liassic deposits in Poland (Except for the Carpathian area) based on microscope examinations. M©moires du Bureau des Recherches G©ologiques et MiniЁres (BRGM) Vol. 75 P. 201- 210
- Marcinkiewicz, T. (1960). Megaspore analysis of Jurassic sediments near Gorzow Slaski Praszha (Cracow-Wielun region). Kwart. geol, 4, 713-33.
- Malinowska, L. (1980). Atlas skamieniałości przewodnich i charakterystycznych. Część 2b, mezozoik/jura. Budowa Geologiczna Polski, 3.
- Marcinkiewicz, T. (1974). Występowanie megaspor w obrębie zaburzonych warstw i liasu w profilu Koszalina. Geological Quarterly, 18(3), 595-601.
- MARCINKIEWICZ, T., FIJAŁKOWSKA-MADER, A. N. N. A., & PIEŃKOWSKI, G. (2014). Poziomy megasporowe epikontynentalnych utworów triasu i jury w Polsce–podsumowanie. Biuletyn Państwowego Instytutu Geologicznego, 457, 15-42.
- Kovach, W. L., & Dilcher, D. L. (1985). Morphology, ultrastructure, and paleoecology of Paxillitriletes vittatus sp. nov. from the mid‐Cretaceous (Cenomanian) of Kansas. Palynology, 9(1), 85-94.
- Marcinkiewicz, T. E. R. E. S. A. (1989). Remarks on agglomerations of megaspores Minerisporites institus Marc. Acta Palaeobotanica, 29, 221-224.
- Dyakowska,J. (1958) The angiospermoid pollen from the Liassic flora in Poland Veroffentlichungen des Geobotanischen Inst.Rubel i N Zurich,Verhandlu,4th Int.Meet,Quat.Botan. Vol. 34 P. 42- 43
- Hemsley, A. R., Collinson, M. E., Kovach, W. L., Vincent, B., & Williams, T. (1994). The role of self-assembly in biological systems: evidence from iridescent colloidal sporopollenin in Selaginella megaspore walls. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 345(1312), 163-173.
- Marcinkiewicz,T. et al. (1960) Age of the Upper Helenow Beds, (Lias), in view of mega and microspore investigations, (Geological section Gorzow Slaski-Praska). [ Wiek Warstw Helenowskich Gornych (Lias) w Przekroju Geologicznym Gorzow Slaski -Praszka w Swietle badan Mega-i Mikrsporowych. ] Kwartalnik Geologiczny (Instytut Geologiczy) Vol. 4 # 2 P. 386- 398
- Ziaja, J. (2006). Lower Jurassic spores and pollen grains from Odrowąż, Mesozoic margin of the Holy Cross Mountains, Poland. Acta Palaeobotanica, 46(1), 3-83.
- Grabowska,I. et al. (1970) Flora from the Lower and Middle Jurassic microflora Geology of Poland. Catalogue of Fossils. Mesozoic Vol. 2 # 2 P. 47- 53
- Migier T. 1978. Nowe stanowiska flory jurajskiej w Lubelskim Zagłębiu Węglowym. Materiały III Naukowej Konferencji Paleontologów poświęconej badaniom regionu górnośląskiego oraz karbonu LZW i GZW. Streszczenia komunikatów: 33–34.Uniwersytet Śląski, Katowice.
- Ruebsam, W., Pieńkowski, G., & Schwark, L. (2020). Toarcian climate and carbon cycle perturbations–its impact on sea-level changes, enhanced mobilization and oxidation of fossil organic matter. Earth and Planetary Science Letters, 546, 116417.
- Samsonowicz, J. (1929). Cechsztyn, trias i lias na północnem zboczu Łysogór.. PIG, Sprawozdania,t. V, z. 1-2
- Szydeł, Z., & Szydeł, R. (1981). Profil utworów liasu na obszarze Lubelskiego Zagłębia Węglowego. Przegląd Geologiczny, 29(11), 568-571.
- Pacyna, G. (2013). Critical review of research on the Lower Jurassic flora of Poland. Acta Palaeobotanica, 53(2), 141-163.