Sorthat Formation
The Sorthat Formation is a geologic formation on the island on Bornholm, Denmark. It is of latest Pliensbachian-Lower Toarcian age. Plant fossils have been recovered from the formation, along with several traces of invertebrate animals. The Sorthat Formation is overlain by fluvial to lacustrine gravels, along with sands and clay along with parts covered by coal beds, that are part of the Aalenian-Bathonian Bagå Formation.[2] In fact the Sorthat Formation was included on most of late SXX studies as the lowermost part of the Bagå Formation, recovering the latest Pliensbachian to lower Aalenian boundary, that was dismissed after compare the geology of the two.[3][4] The Sorthat strata recover a mostly marginal deltaic to marine unit, where large streams fluctuated to the east where a large river system was stablished at the start of the Toarcian.[2] At the northwest the local Vulcanism, that started on the lower Pliensbachian, extended along the North Sea and mostly from southern Sweden.[5] At this time, the Central Skåne Volcanic Province and the Egersund Basin expulsed most of his strata, with influences on the local tectonics.[5] The Egersund Basin has abundant fresh prophyritic Nephelinite lavas and dykes of lower Jurassic Age, with a composition nearly equal to those found on the clay pits. That reveals the translation of strata from the Continental margin by large fluvial channels, that ended on the sea deposits of the Ciechocinek Formation Green series.[5]
| Sorthat Formation Stratigraphic range: Latest Pliensbachian to Early-mid Toarcian ~183–178 Ma | |
|---|---|
 Mostly of the Strata of the Formation is found on Cliffs on the Southern Part of the Island | |
| Type | Geological formation |
| Underlies | Bagå Formation |
| Overlies | Rønne & Hasle Formations |
| Thickness | 3–200 m (9.8–656.2 ft)[1] |
| Lithology | |
| Primary | Claystone, sandstone[1] |
| Location | |
| Region | Bornholm |
| Country | |
| Type section | |
| Named for | Sorthat-Muleby, Bornholm |
| Named by | Gry (as part of the Bagå Formation) [2] |
| Year defined | 1969 |
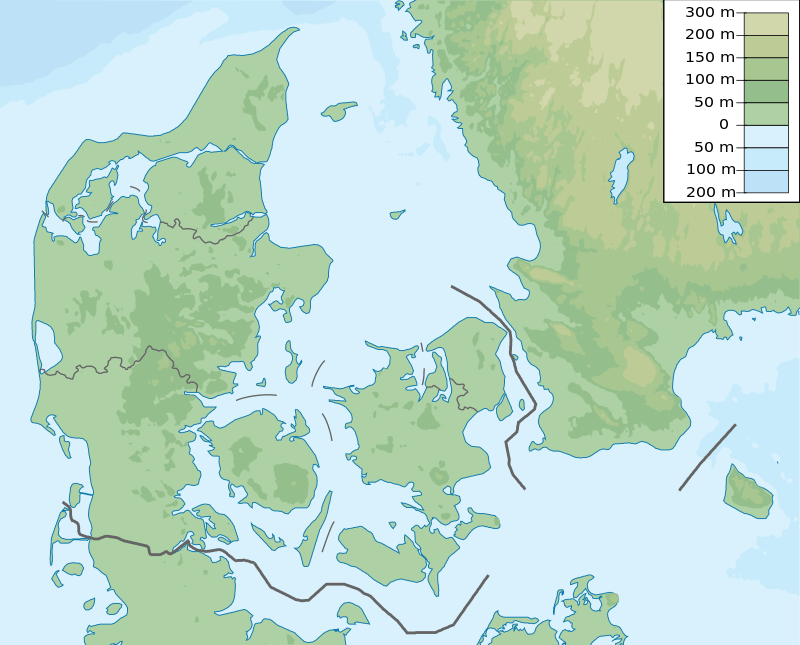 Sorthat Formation (Denmark) | |
Stratigraphy
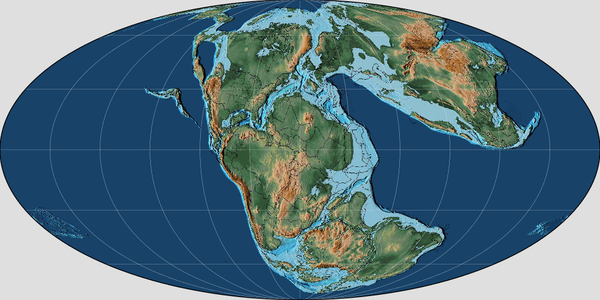
On Bornholm, the lower-middle Jurassic succesion is composed of the Rønne (Hettangian-Sinemurian), Hasle (Lower-late Pliensbachian), Sorthat and Bagå Formations. The major Pliensbachian–Bathonian coal-bearing Clays and Sands that overlie the Lower Pliensbachian Hasle Formation are distributed between the to the new Sorthat Formation and the Bagå Formation.[1] The Sorthat Formation is the regional equivalent of the Ciechocinek Formation on Baltic Germany and Poland, the Fjerritslev Formation on the Danish Basin, the Rya Formation on Scania.[1] The Sorthat Formation beds where referred originally to the Levka Beds, Sorthat and Bagå beds.[2] The major section of the Formation is the Korsodde coastal section located on the south-west part of the Island.[2] A detailed stratigraphic interpretation of the beds has been difficult to achieve due to the complicated block faulting, but specially due to the absence of marine fossils and distinct marker beds.[2] Using the Megaspore contents, the rocks where dated originally as Middle Jurassic, with the Levka and Sorthat beds being roughly contemporaneous, and the Bagå beds being possibly slightly younger. Latter the Bagå Formation was limited, including the Coal Clays of the Levka Beds, along with the also Coal Dominat beds of the Korsodde and Onsbæk sections.[3] With more advanced Palynological studies recovered from locations such as the Levka-1 core-well and the Korsodde section Upper Pliensbachian strata appeared.[6][7] As at the time several Megaspores collected where found to be common on both the Bagå Formation and Sorthat beds, implying at least the presence of Toarcian-Aalenian strata.[3] Although it was difficult to set a concrete age for the Megaspore-bearing strata.[8] With both, the palynological–sedimentological study of all available exposures and cores from the Lower–Middle Jurassic lead to the revelation that the Hasle Formation (Lower-Middle Pliensbachian) are covered by a succession referable to both the Levka and Sorthat Beds, that are composed mostly by bioturbated Sands, Heteroliths and Clays along with abundant Coal seams containing relatively diverse brackish-marine dinoflagellate assemblages that are indicative of the upper Pliensbachian, Toarcian and possibly lower Aalenian strata.[6] While the upper strata is covered by the fluvial gravels and sands, along with lacustrine clays, carbonaceous clays and coals belonging to the Bagå Formation.[1]
Lithology
The Sorthat Formation has a highly variable lithology.[1] The main core studied from the rocks, the Levka-1 well reveal first a sharp-based, fining-upwards units, 3–14 m thick, consisting of coarse-grained, occasionally pebbly Sand, overlain by muddy, with Coal and Mica, fine- to medium-grained sand, that is laminated to homogeneous Clay and coal seams with roots.[1] On most of the strata there is a common Parallel Lamination with subordinate Cross-bedding, Cross-lamination and Flaser lamination.[1] There are abundant Large plant fragments and small Quartz. Related to this level, there is a clear absence of Marine palynomorphs are not found, as a main probe that this level was deposited on a coastal or Delta plain with fluvial channels, Lakes and Swamps.[6] This is also related to the most recent finds on the German realm of the Ciechocinek Formation, where a large Deltaic System ended. The large Toarcian–Bajocian deltaic systems locally where the shoreline influenced by the vicinity between brackish to freshwater and continental biofacies.[9][10] The North German Basin shows that on approximately 14.4 m.a, four third-order relative sea-level fluctuations led the subsequent formation of four individual delta generations in the Bifrons-Thouarsense (Toarcian), Murchisonae-Bradfordensis (Aalenian) and Humpresianum-Garatiana (Bajocian).[9] The Toarcian section was dominated by regressive elongated river-dominated deltas, where due to the fall of the sea level the south to southwest directed delta progradation between the Lower-Upper Toarcian, that was deposited as 40 m of deltaic successions, found on places like Prignitz (East) and Brandenburg (North).[9] Most of the Palynomorphs found on the Toarcian strata are connected with the ones found on the Sorthat Formation.[9] With nearly 40 m thick, the upper section of the formation is composed mostly by a series of cross-bedded, cross-laminated, wave-rippled and bioturbated sand and heteroliths with sporadic Syneresis cracks, Pyrite nodules, the ichnofossils Planolites isp. and Teichichnus isp. and brackish-marine palynomorphs, mostly dinoflagellates.[1] This upper part has a strata more common to be deposited on nearshore environments with abundant lagoons, coastal lakes and fluvial channels with the clean sand at the top probably representing a marine shoreface.[1] The Korsodde Section, with 93 m thick is composed mostly by coarse grained Sands with cross-bedding and parallel lamination, being mostly of Black due to an abundant organic debris.[1] This Section has been interpreted as part of the large local fluvial system, probably as a series of minor fluvial channels, that where connected with coastal lakes and lagoons, where riparian vegetation was relatively abundant, judging by the presence of megaflora remains and palynomorphs.[1] Small ichnofossil burrows and larger burrows, including Diplocraterion isp. are common, interpreting that there was at least one subunit that was the fill of an estuarine channel.[1] The uppermost part of the formation in the Korsodde section consists of fine-grained, sands with cross stratification and parallel-lamination, yellowish–brown color, along with Sandstones with thin bioturbated and wave-rippled heterolithic beds.[1]
Flora
One of the Most Complete Floras found on All Europe on the Pliensbachian-Toarcian Boundary, and among all the Jurassic Palynologycal deposits found Worldwide.[4][7][8][11]
Environment
Due to a Late Pliensbachian marine regression, deposition of coal-bearing strata on the Sorthat Formation was resumed on Bornholm until an Early Toarcian transgression terminated peat formation.[12] The two main deposits of the formation, the Levka-1 Well and the lower part of the Korsodde Section where deposited on an environment influenced by the sea, being the Levka location populated by Lagoons, lakes, channels and low fluvial areas.[12] Inertinite has been recovered from the Coal-Bearing levels of the formation, where the Palynology shows that the mire vegetation may have been dominated by gymnospermous plants and a secondary proportion of ferns characterised by the genera Dicksonia or Coniopteris and the family Osmundaceae.[12] O several coal seams there where found several Biomolecules, where Euulminite and Attrinite were the most abundant huminite macerals recovered.[13] The Levka-1 well section represents fluvial channels, floodplain areas with shallow lakes and lagoons, and small crevasse deltas, with abundant coalified wood fragments and stems, being most of them found associated to sandy channel fills and on heavily rooted crevasse and lake deposits in shallow inter-fluvial areas.[14] On the Toarcian at Bornholm strata indicates a warm, humid climate suggested by the large number of plant species from interconnected Jameson Land, and thin cutinised leaves of Podozamites and Equisetales comparable in size to modern subtropical bamboos are thought to reflect favourable conditions for plant growth.[14] There is abundant Coal, which indicates that wildfires occurred in the bog.[14] Wood particles from this section, both charcoalified and unburned (coalified), with a rounded and worn nature on many particles, that implies the influence of greater transportation energies.[15] The Toarcian Erratics from Northern Germany are assigned to Southern Sweden and also to the Sorthat Formation, being sugested as the source area of the Ahrensburg erratics.[16] On the Toarcian, the facies on Sweden the sketchy facies information suggests a general lateral coarsening of the lithofacies on Helsingborg (Rya Formation) and the Sorthat Formation, where there is the probe of the presence of a large deltaic system that fluctuated from the SE of Sweden and Bornholm, that ended on a prodeltaic facies belt stretching from Vorpommern to Mecklenburg Bay.[16]
Coal
The Levka- 1 well has a core of approx. 150 m on the Sorthat Formation, covering the underliying marine strata of the Hasle Formation.[17] The lower part includes 112 m with the Coal along sand and clay. There if an abundant presence of large, coalified, wood fragments and stems.[17] The Coal bearing strata of the Levka-1 are interpreted as fluvial channel fills, with active channel deposition followed by abandonment and a passive phase of clay deposition, gradual overgrowth and change into a peat-forming environment.[17] Clay and coal seams present along this strata have abundant rootlets and a non-marine palynomorph assemblage dominated by spores and pollen, interpreted as representing flood plain areas with shallow Lakes, small crevasse Deltas and Swamps. Some section have wave ripples, wavy and flaser bedding, bioturbation and transported Equisetites stems, that are interpreted as the sediment fill of a local lagoon, deposited on a transgressive shoreline with a series of lagoon succesions.[17] Levka-1 coal contain hard, black coal and are very similar petrographically, with Huminite on the major part of the seams, with some seams having up to 90% of Huminite. There is a dominance of macerals from detrital organic matter (Humodetrinite) over macerals derived from more woody material (Humotelinite).[17] Gelinite appears as the most common component of the samples, followed by Huminite.[17]
The Korsodde section is interpreted as representing an small coastal series of lakes and protected lagoons, where are recovered at least 6 coal seams occur. Above the marine strata of the Toarcian Tranggression strata with abundant Clay fine-grained Sand, Silt, that contains transported, coalified pieces of wood wood, pyrite nodules, rootlets and a diverse miospore assemblage, where there is abundance of the marine dinoflagellate Mendicodinium reticulaturn.[17] On this coal Seams Huminite maceral group comprises the majority of the organic matter, where humotelinite dominates over humodetrinite maceral. Eu-ulminite and Densinite are the most prominent macerals.[17]
Marine Palynology
On the Lower Jurassic of Bornholm there where several succesions of nearshore Peat Formations with Dinoflajellates.[17] Coal-bearing strata were deposited in an overall coastal plain environment during the Hettangian-Sinemurian and then during the Early Pliensbachian was interrupted until the late Pliensbachian-Lowermost Toarcian due to a Sea regression.[17]
Color key
|
Notes Uncertain or tentative taxa are in small text; |
| Genus | Species | Stratigraphic position | Material | Notes | Images |
|---|---|---|---|---|---|
|
|
|
Type Genus of the Botryococcaceae inside Trebouxiales. A colonial green microalga related with freshwater and brackish ponds and lakes around the world, where it often can be found in large floating masses. Sorthat Formation Botryococcus lived on an environment interpreted as a coastal lake, permanently vegetated and shallow that was occasionally flooded by the sea. |
 Pustulina | |
|
Mancodinium[17] |
|
|
|
A Dinoflajellate, type Genus of the Mancodinioideae. |
|
|
Mendicodinium[17] |
|
|
|
A Dinoflajellate, member of the family Gonyaulacales. |
|
|
Nannoceratopsis[17] |
|
|
|
A Dinoflajellate, member of the family Nannoceratopsiaceae. It is a genus related with Marine deposits. |
|
Terrestrial Palynology
In Early Toarcian carbonates local bulk organic matter, and wood fragments has been related to carbon cycle perturbations, helping to know the reaction of the Continental Biota to the TAOE that happened along contemporaneous large scale volcanism.[18] There where several changes on the woody vegetation in the wood-derived carbon, with pollen assem-blages are dominated by Taxodiaceae pollen types such Cerebropollenites associated with Cycad pollen types (Chasmatosporites) and the Cheirolepidiaceous Corollina, reflecting a warming transition from a wet fern dominated landscape to seasonally dry vegetation.[18] Is also common to found wood from a near-shore setting on the eastern Danish Basin, with two sets: macroscopic wood, recognizable to the naked eye, up to branch-sized; and microscopic wood (0.25 to 1 mm average dimension).[19] Apart from wood and leaf cuticles there is little organic matter present in the section—the sediments comprise sand, silt and mud—and thus there is no possibility of contamination by marine carbon.[19]
Color key
|
Notes Uncertain or tentative taxa are in small text; |
| Genus | Species | Stratigraphic position | Material | Notes | Images |
|---|---|---|---|---|---|
|
|
|
Affinities with the Rhyniophyta. It is related to the basal Plants of the Devonian, such as the genus Rhynia. Can be Spores from a Relict Taxon. |
 | |
|
|
|
Affinities with the Lycophyta. It comprises the main Megaspore zonation of the Toarcian of Denmark, being the most abundant spore found. |
||
|
|
|
Affinities with the Lycophyta. |
||
|
|
|
Affinities with the Lycophyta. Lycopod Spores, related with Herbaceous to arbustive flora common on humid environments |
||
|
|
|
Affinities with the Lycophyta. Lycopod Spores, related with Herbaceous to arbustive flora common on humid environments |
||
|
|
|
Affinities with the Lycophyta. Lycopod Spores, related with Herbaceous to arbustive flora common on humid environments |
||
|
|
|
Affinities with the Lycophyta. Lycopod Spores, related with Herbaceous to arbustive flora common on humid environments |
||
|
|
|
Affinities with the Lycophyta. Lycopod Spores, related with Herbaceous to arbustive flora common on humid environments |
||
|
|
|
Affinities with the Lycophyta. Lycopod Spores, related with Herbaceous to arbustive flora common on humid environments |
||
|
|
|
Affinities with the Lycopsida inside Lycophyta. Lycopod Spores, related with Herbaceous to arbustive flora common on humid environments |
||
|
|
|
Affinities with the Lycopsida inside Lycophyta. Lycopod Spores, related with Herbaceous to arbustive flora common on humid environments |
||
|
|
|
Affinities with the Lycopsida inside Lycophyta. Assumed to be the products of heterosporous lycopods. |
||
|
|
|
Affinities with the Lycopsida inside Lycophyta. Lycopod Spores, related with Herbaceous to arbustive flora common on humid environments |
 | |
|
|
|
Affinities with the Lycopsida inside Lycophyta. Lycopod Spores, related with Herbaceous to arbustive flora common on humid environments |
||
|
|
|
Affinities with the Lycopsida inside Lycophyta. Lycopod Spores, related with Herbaceous to arbustive flora common on humid environments |
||
|
|
|
Affinities with the Lycopsida inside Lycophyta. Lycopod Spores, related with Herbaceous to arbustive flora common on humid environments |
||
|
|
|
Affinities with the Lycopodiales inside Lycophyta. Low floor Spores, related with Herbaceous to arbustive flora common on humid environments |
||
|
|
|
Affinities with the Lycopodiales inside Lycophyta. Low floor Spores, related with Herbaceous to arbustive flora common on humid environments |
||
|
Lycopodiumsporites[22] |
|
|
|
Affinities with the Lycopodiaceae inside Lycophyta. Low floor Spores, related with Herbaceous to arbustive flora common on humid environments |
|
|
|
|
Affinities with the Isoetales inside Lycophyta. Low floor Spores, related with Herbaceous to arbustive flora common on humid environments |
 | |
|
|
|
Affinities with the Isoetales inside Lycophyta. Low floor Spores, related with Herbaceous to arbustive flora common on humid environments |
||
|
|
|
Affinities with the Bryophyta. Moss Spores, related to high Humid Environments |
.jpg) | |
|
|
|
Affinities with the Bryophyta. Moss Spores, related to high Humid Environments |
||
|
|
|
Affinities with the Bryophyta. Moss Spores, related to high Humid Environments |
||
|
|
|
Affinities with the Bryophyta. Moss Spores, related to high Humid Environments |
||
|
|
|
Affinities with the Bryophyta. Moss Spores, related to high Humid Environments |
||
|
|
|
Affinities with the Bryophyta. Moss Spores, related to high Humid Environments |
||
|
|
|
Affinities with the Bryophyta. Moss Spores, related to high Humid Environments |
||
|
|
|
Affinities with the Bryophyta. Moss Spores, related to high Humid Environments |
||
|
|
|
Affinities with the Bryophyta. Moss Spores, related to high Humid Environments |
||
|
|
|
Affinities with the Bryophyta. Moss Spores, related to high Humid Environments |
||
|
|
|
Affinities with the Bryophyta. Moss Spores, related to high Humid Environments |
||
|
|
|
Affinities with the Bryophyta. Moss Spores, related to high Humid Environments |
||
|
|
|
Affinities with the Bryopsida inside Bryophyta. Moss Spores, related to high Humid Environments |
 | |
|
|
|
Affinities with the Bryopsida inside Bryophyta. Moss Spores, related to high Humid Environments |
||
|
|
|
Affinities with the Sphaerocarpales inside Marchantiopsida. Liverwort Spores, related to high Humid Environments |
 | |
|
|
|
Affinities with the Calamitaceae inside Equisetales. Herbaceous Flora related to high humid environments |
||
|
|
|
Affinities with the Selaginellaceae. Herbaceous Flora related to high humid environments |
 | |
|
|
|
Affinities with the Selaginellaceae. Herbaceous Flora related to high humid environments |
||
|
|
|
Affinities with the Selaginellaceae. Herbaceous Flora related to high humid environments |
||
|
|
|
Affinities with the Selaginellaceae. Herbaceous Flora related to high humid environments |
||
|
|
|
Affinities with the Selaginellaceae. Herbaceous Flora related to high humid environments |
||
|
|
|
Affinities with the Selaginellaceae. Herbaceous Flora related to high humid environments |
||
|
|
|
Affinities with the Selaginellaceae. Herbaceous Flora related to high humid environments |
||
|
|
|
Affinities with the Selaginellaceae. Herbaceous Flora related to high humid environments |
||
|
|
|
Affinities with the Selaginellaceae. Herbaceous Flora related to high humid environments |
||
|
|
|
Affinities with the Selaginellaceae. Herbaceous Flora related to high humid environments |
||
|
|
|
Affinities with the Gymnospermophyta. Non concreted family affinities |
||
|
|
|
Affinities with the Gymnospermophyta. Non concreted family affinities |
||
|
|
|
Affinities with the Gymnospermophyta. Non concreted family affinities |
||
|
|
|
Affinities with the Gymnospermophyta. Non concreted family affinities |
||
|
|
|
Affinities with the Gymnospermophyta. Non concreted family affinities |
||
|
|
|
Affinities with the Gymnospermophyta. Non concreted family affinities |
||
|
|
|
Affinities with the Gymnospermophyta. Non concreted family affinities |
||
|
|
|
Affinities with the Gymnospermophyta. Non concreted family affinities |
||
|
|
|
Affinities with the Gymnospermophyta. Non concreted family affinities |
||
|
|
|
Affinities with the Gymnospermophyta. Non concreted family affinities |
||
|
|
|
Affinities with the Filicales. Fern Spores from lower Herbaceous flora |
||
|
|
|
Affinities with the Filicales. Fern Spores from lower Herbaceous flora |
||
|
|
|
Affinities with the Ophioglossaceae inside Filicales. Fern Spores from lower Herbaceous flora |
| |
|
|
|
Affinities with the Pteridophyta. Fern Spores from lower Herbaceous flora |
||
|
|
|
Affinities with the Pteridophyta. Spores from abundant water environments ferns. |
||
|
|
|
Affinities with the Pteridophyta. Fern Spores from lower Herbaceous flora |
||
|
|
|
Affinities with the Pteridophyta. Fern Spores from lower Herbaceous flora |
||
|
|
|
Affinities with the Pteridophyta. Fern Spores from lower Herbaceous flora |
||
|
|
|
Affinities with the Pteridopsida. Fern Spores from lower Herbaceous flora |
||
|
|
|
Affinities with the Pteridopsida. Fern Spores from lower Herbaceous flora |
||
|
|
|
Affinities with the Pteridopsida. Fern Spores from lower Herbaceous flora |
||
|
|
|
Affinities with the Pteridopsida. Fern Spores from lower Herbaceous flora |
||
|
|
|
Affinities with the Pteridopsida. Fern Spores from lower Herbaceous flora |
||
|
|
|
Affinities with the Pteridopsida. Fern Spores from lower Herbaceous flora |
||
|
|
|
Affinities with the Pteridopsida. Fern Spores from lower Herbaceous flora |
||
|
|
|
Affinities with the Callistophytaceae inside Pteridospermatophyta. Spores from large arboreal to arbustive ferns |
||
|
|
|
Affinities with the Peltaspermaceae inside Pteridospermatophyta. Spores from large arboreal to arbustive ferns |
||
|
|
|
Affinities with the family Lygodiaceae inside Polypodiopsida. Climbing Fern Spores |
 | |
|
|
|
Affinities with the Schizaeaceae inside Polypodiopsida. Fern Spores from lower Herbaceous flora |
 | |
|
Undulatisporites[22] |
|
|
|
Affinities with the Schizaeaceae inside Polypodiopsida. Fern Spores from lower Herbaceous flora |
|
|
Osmundacidites[22] |
|
|
|
Affinities with the Osmundaceae inside Polypodiopsida. Near Fluvial currents ferns, reted to the modern Osmunda Regalis. |
 |
|
|
|
Affinities with the Pteridaceae inside Polypodiopsida. Fern Spores from lower Herbaceous flora |
.jpg) | |
|
|
|
Affinities with the genus Ceratopteris (Parkerioideae) inside Polypodiopsida. Spores from aquatic or subaquatic ferns of moderate size. Are abundant on the layers near fluvial deposition, probably associated to large underwater colonies of ferns on local freshwater deposits. |
 | |
|
|
|
Affinities with the genus Dicksoniaceae inside Polypodiopsida. Tree Fern Spores |
_C.Chr._by_Jason_Hollinger_001.jpg) | |
|
|
|
Affinities with the Gleicheniales inside Polypodiopsida. Fern Spores from lower Herbaceous flora |
 | |
|
|
|
Affinities with the Marattiaceae inside Polypodiopsida. Fern Spores from lower Herbaceous flora |
.jpg) | |
|
|
|
Affinities with the Matoniaceae inside Polypodiopsida. Fern Spores from lower Herbaceous flora |
 | |
|
|
|
Affinities with the Polypodiaceae inside Polypodiopsida. Fern Spores from lower Herbaceous flora |
 | |
|
|
|
Affinities with the Bennettitales. Arbustive Cycad-Like Flora |
||
|
|
|
Affinities with the Bennettitales. Arbustive Cycad-Like Flora |
||
|
|
|
Spores assigned to the genus Bjuvia, an arboreal member of Cycadales inside Cycadophyta |
 | |
|
|
|
Affinities with the Coniferophyta. Conifer Pollen from medium to large Arboreal Plants |
||
|
|
|
Affinities with the Coniferales. Conifer Pollen from medium to large Arboreal Plants |
||
|
|
|
Affinities with the Coniferales. Conifer Pollen from medium to large Arboreal Plants |
||
|
|
|
Affinities with the Coniferales. Conifer Pollen from medium to large Arboreal Plants |
||
|
|
|
Affinities with the Pinopsida inside Coniferales. Conifer Pollen from medium to large Arboreal Plants |
 | |
|
|
|
Affinities with the Pinopsida inside Coniferales. Conifer Pollen from medium to large Arboreal Plants |
||
|
|
|
Affinities with the Protopinaceae inside Pinopsida. Conifer Pollen from medium to large Arboreal Plants |
||
|
|
|
Affinities with the Pinaceae inside Pinopsida. Conifer Pollen from medium to large Arboreal Plants |
||
|
|
|
Affinities with the Pinaceae inside Pinopsida. Conifer Pollen from medium to large Arboreal Plants |
||
|
|
|
Affinities with the Podocarpaceae inside Pinopsida. Conifer Pollen from medium to large Arboreal Plants |
_002.jpg) | |
|
|
|
Affinities with the Podocarpaceae inside Pinopsida. Conifer Pollen from medium to large Arboreal Plants |
||
|
|
|
Affinities with the Cheirolepidiaceae inside Pinopsida. Conifer Pollen from medium to large Arboreal Plants |
||
|
|
|
Affinities with the Cheirolepidiaceae inside Pinopsida. Conifer Pollen from medium to large Arboreal Plants, this palynomorph can compose up to 95 % of an assemblage and is correlated with Toarcian carbon cycle anomalies including the oceanic anoxic event.[22] |
||
|
|
|
Affinities with the Araucariaceae inside Pinales. Conifer Pollen from medium to large Arboreal Plants |
| |
|
|
|
Affinities with the Monocotyledoneae. Possible Pollen from primigenial Angiosperm Plants. Alternatively, can be Pollen from Bennetitales. |
||
|
|
|
Affinities with the Magnoliidae inside Magnoliopsida. Possible Pollen from primigenial Angiosperm Plants |
||
Plant Remains
Color key
|
Notes Uncertain or tentative taxa are in small text; |
| Genus | Species | Stratigraphic position | Material | Notes | Images |
|---|---|---|---|---|---|
|
|
|
Affinities with Equisetaceae inside Equisetales. Equisetalean Stems, related that are also found on the Hettangian strata along Skane, Sweden. On the Lagoonar sections there is correlation between bioturbation and transported Equisetites stems.[24] Local Equisetales obtained a considerable size, comparable to modern subtropical bamboos, close to lakes and in the wettest environments.[17] |
||
|
|
|
Affinities with Calamitaceae inside Equisetales. Equisetalean Stems, related that are also found on same age strata along Skane, Sweden. Based on analogies with morphologically similar extant Equisetum species, it is interpreted to represent a plant of consistently moist habitats, such as marshes, lake margins or forest understorey, developed normally dense thickets. |
 Neocalamites specimen from the Sorthat Formation | |
|
|
|
Incertae Ordinis inside Pteridophyta. Spiropteris is the name given to the fossil of a fern leaf before opening, when it is still coiled. |
||
|
|
|
Affinities with Caytoniaceae inside Pteridospermatophyta. Related with the Cycadophytes present in the Rhaetic flora of Sweden. |
||
|
|
|
Affinities with Caytoniaceae inside Pteridospermatophyta. Less common that other Arboreal Plants |
||
|
|
|
Affinities with Osmundaceae inside Osmundales. Related with species commonly reported from the Triassic–Jurassic of southern Sweden. |
Cladophlebis nebbensis specimen | |
|
|
|
Affinities with Dicksoniaceae inside Cyatheales. The Lund-material is dominated by ferns belonging to the genus Eboracia (28 specimens of E. lobifolia and 14 of E. sp.). Eboracia sp. has considerably smaller pinnules than lobifolia. |
||
|
|
|
Affinities with Dicksoniaceae inside Cyatheales. Common cosmopolitan Mesozoic Tree fern genus. |
||
|
|
|
Affinities with Dipteridaceae inside Polypodiales. Specimens from the same species have been found on the same age Höör Sandstone at southern Sweden. |
||
|
|
|
Affinities with Dipteridaceae inside Polypodiales. Dictyophyllum is a common Dipteridacean genus of the mid-Mesozoic |
 Dictyophyllum nilssonii specimen | |
|
|
|
Affinities with Williamsoniaceae inside Bennettitales. Insufficient and incomplete material prevents certain allocation to that species. |
.jpg) Otozamites specimen | |
|
|
|
Affinities with Ginkgoaceae inside Ginkgoales. Seven species assigned to either Ginkgo or Ginkgoites have been reported from the Latest Triassic to middle Jurassic strata of southern Sweden. |
||
|
|
|
Affinities with Ginkgoaceae inside Ginkgoales. Unlike other Plants specimens from the locations is linked more to Middle Jurassic Flora |
||
|
Czekanowskia[27] |
|
|
|
Affinities with Czekanowskiales inside Ginkgoales. This Genus is related to flora from the Rhaetian-Hettangian boundary of Jameson Land, but also present on Romania. |
|
|
Solenites[27] |
|
|
|
Affinities with Czekanowskiales inside Ginkgoales. This species was described on the basis of individuals collected in Greenland, from the Triassic-Jurassic boundary. |
|
|
Hartzia[27] |
|
|
|
Affinities with Czekanowskiales inside Ginkgoales. Linked to the Lower Liassic Flora from Greenland. |
|
|
|
|
Affinities with Araucariaceae or Cheirolepidiaceae inside Pinales. P. steenstrupi is preferred as this species is characterised by relatively broad leaves inserted at high angles to the stem. P. peregrinum has been found on the Hettangian Ronne Formation associated with Cheirolepidaceous wood, Brachyoxylon rotnaensis (Simplicioxylon). |
||
|
|
|
Affinities with Araucariaceae or Cheirolepidiaceae inside Pinales. Is related to Hettangian axis found on Scania, Sweden |
||
|
Dactyletrophyllum[23] |
|
|
|
Affinities with Cheirolepidiaceae inside Pinales. Is related to with other representatives of the genus of the Toarcian of Italy and Lower Jurassic of Israel. Spheripollenites co-occurs with cuticles of Dactyletrophyllum ramonensis, and after a test of relationships it was found a highly significant correlation may suggest that S. psilatus is produced by the conifer genus Dactyletrophyllum.[23] |
|
|
Podozamites[14] |
|
|
|
Affinities with Podocarpaceae inside Pinales. The local Podozamites show a rather great range of Growth, reflecting Tropical to subtropical conditions. |
|
|
Cyparissidium[27] |
|
|
|
Affinities with Taxodiaceae inside Pinales. It matches with the Middle Jurassic Cyparissidium blackii from Yorkshire, England. |
|
Fauna
Ichnofossils
| Genus | Species | Location | Material | Notes | Images |
|---|---|---|---|---|---|
|
|
Burrowing and track ichnofossils. |
Burrow-like ichnofossils. It is referred to vermiform deposit-feeders, mainly Polychaetes, producing active Fodinichnia. It is controversial, since is considered a strictly a junior synonym of Palaeophycus.[29] |
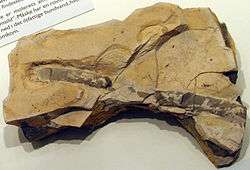 Example of Planolites fossil | |
|
Palaeophycus[28] |
|
|
Burrowing and track ichnofossils. |
Burrow-like ichnofossils. They occur in different size classes, 3, 5 and 10 mm in diameter. |
Example of Palaeophycus fossil |
|
Bornichnus[28] |
|
|
Burrowing and track ichnofossils. |
Burrow-like ichnofossils. Exclusive from the Formation, Bornichnus differs from Palaeophycus Hall in its tangled, contorted morphology and abundant branching. Similar complicated burrow systems are produced by the polychaete Capitomastus cf. aciculatus. |
|
|
|
Burrowing and track ichnofossils. |
Burrow-like ichnofossils. Ichnofossils done by organisms advancing along the bottom surface. Very narrow, vertical or subvertical, slightly winding unlined shafts filled with mud. |
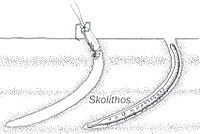 Skolithos ichnofosil reconstruction, with possible fauna associated | |
|
Cylindrichnus[28] |
|
|
Burrowing and track ichnofossils. |
Burrow-like ichnofossils. Cylindrichnus isp. was found only in the uppermost part of the section, and probably represents Polychaete Burrows.[30] |
|
|
|
Burrowing and track ichnofossils. |
Burrow-like ichnofossils. They are believed to be Fodinichnia, with the organism adopting the habit of retracing the same route through varying heights of the sediment, which would allow it to avoid going over the same area. |
 Example of Teichichnus fossil | |
|
|
Burrowing and track ichnofossils |
Burrow-like ichnofossils, that can be related to Crustaceans, Annelids and Fishes. |
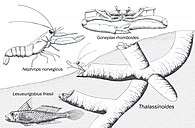 Thalassinoides burrowing structures, with modern related fauna, showing the ecological convergence and the variety of animals that left this Ichnogenus. | |
|
|
Burrowing and track ichnofossils |
Burrow-like ichnofossils. Interpreted as the feeding burrow of a sediment-ingesting animal.[31] A more recent study has find that Scoloplos armiger and Heteromastus filiformis, occurring in the German Wadden Sea in the lower parts of tidal flats, make burrows that are homonymous with numerous trace fossils of the ichnogenus.[32] |
Illustration of Chondrites bollensis | |
|
|
Burrowing and track ichnofossils. |
Burrow-like ichnofossils. Vertical or oblique complex trace fossil composed of a bunch of spindle-shaped structures and associated tubes, typical of a restricted environment (?estuarine/lagoonal). |
||
|
|
Burrows and associated traces |
Burrow-like ichnofossils. Most Diplocraterion show only protrusive spreit, like the local ones, produced under predominantly erosive conditions where the organism was constantly burrowing deeper into the substrate as sediment was eroded from the top. It can be Made by Crustaceans, Annelids od toher benthic fauna.[28] |
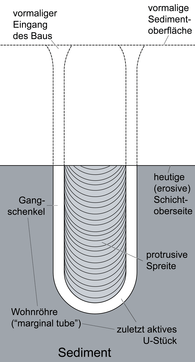 Diplocraterion parallelum diagram | |
References
- Michelsen, O., Nielsen, L. H., Johannessen, P. N., Andsbjerg, J., & Surlyk, F. (2003). Jurassic lithostratigraphy and stratigraphic development onshore and offshore Denmark. Geological Survey of Denmark and Greenland (GEUS) Bulletin, 1, 145-216.
- Gry, H., Jørgart, T., & Poulsen, V. (1969). Geologi pa Bornholm. Mineralogisk Mus.
- Gravesen, P. (1982). lithostratigraphy and sedimentary evolution of the Triassic, Jurassic and Lower Cretaceous of Bornholm, Denmark.
- Gry, H. E. L. G. E. (1969). Megaspores from the Jurassic of the island of Bornholm, Denmark. Meddelelser fra Dansk Geologisk Forening, 19, 69-89.
- Bergelin, I., Obst, K., Söderlund, U., Larsson, K., & Johansson, L. (2011). Mesozoic rift magmatism in the North Sea region: 40 Ar/39 Ar geochronology of Scanian basalts and geochemical constraints. International Journal of Earth Sciences, 100(4), 787-804.
- Nielsen, L.H. 1987: Progress report 1.1.1988. Biostratigraphy and organic geochemistry of the Mesozoic on Bornholm. DGU 215 confidential report 31, 35 pp. Copenhagen: Geological Survey of Denmark.
- Koppelhus, E.B. 1988: Catalogue of spores and pollen from the Lower–Middle Jurassic Bagå Formation on Bornholm, Denmark. DGU confidential report 21, 42 pp. Copenhagen: Geological 214 Survey of Denmark
- Koppelhus, E.B. & Batten, D.J. 1992: Megaspore assemblages from the Jurassic and lowermost Cretaceous of Bornholm, Denmark. Danmarks Geologiske Undersøgelse Serie A 32, 81 pp
- Zimmermann, J., Franz, M., Schaller, A., & Wolfgramm, M. (2017). The Toarcian-Bajocian deltaic system in the North German Basin: Subsurface mapping of ancient deltas-morphology, evolution and controls. Sedimentology, 65(3), 897–930. doi:10.1111/sed.12410
- Barth, G., Pieńkowski, G., Zimmermann, J., Franz, M., & Kuhlmann, G. (2018). Palaeogeographical evolution of the Lower Jurassic: high-resolution biostratigraphy and sequence stratigraphy in the Central European Basin. Geological Society, London, Special Publications, 469(1), 341-369.
- Hoelstad,T. (1985) Palynology of the Uppermost Lower to Middle Jurassic strata on Bornholm, Denmark. Geological Society of Denmark, Bulletin (Meddelelser Fra Dansk Geologisk Forening) Vol. 34 P. 111- 132
- PETERSEN, H.I., NIELSEN, L.H., KOPPELHUS, E.B., and SØRENSEN, H.S., 2003, Early and Middle Jurassic mires of Bornholm and the Fennoscandian Border Zone: A comparison of depositional environments and vegetation: Geological Survey of Denmark and Greenland Bulletin, v. 1, p. 631–656
- Petersen, H. I. (1993). Petrographic facies analysis of Lower and Middle Jurassic coal seams on the island of Bornholm, Denmark. International Journal of Coal Geology, 22(3-4), 189-216.
- Petersen, H. I., Nielsen, L. H., Koppelhus, E. B., & Sørensen, H. S. (2003). Early and Middle Jurassic mires of Bornholm and the Fennoscandian Border Zone: a comparison of depositional environments and vegetation. Geological Survey of Denmark and Greenland (GEUS) Bulletin, 1, 631-656.

- Crawford, A. J. (2015). Understanding fire histories: the importance of charcoal morphology.
- Sachs, S., Hornung, J. J., Lierl, H.-J., & Kear, B. P. (2016). Plesiosaurian fossils from Baltic glacial erratics: evidence of Early Jurassic marine amniotes from the southwestern margin of Fennoscandia. Geological Society, London, Special Publications, 434(1), 149–163. doi:10.1144/sp434.14
- Petersen,H.I. et al. (1995) Controls on Peat accumulation and depositional environments of a Coal-bearing Coastal Plain succession of a Pull-Apart Basin; a petrographic, geochemical and sedimentological study, Lower Jurassic, Denmark International Journal of Coal Geology Vol. 27 # 2 P. 99- 129
- Wade-Murphy, J., Kuerschner, W. M., & Hesselbo, S. P. (2006). Early Toarcian vegetation history from the Korsodde Section of Bornholm (Denmark) and its possible impact on terrestrial carbon isotope records. In 7 th European Palaeobotany Palynology Conference, Prague (p. 153).
- Hesselbo, S. P., Gröcke, D. R., Jenkyns, H. C., Bjerrum, C. J., Farrimond, P., Bell, H. S. M., & Green, O. R. (2000). Massive dissociation of gas hydrate during a Jurassic oceanic anoxic event. Nature, 406(6794), 392-395.
- Seidenkrantz,M.S. et al. (1993) Biostratigraphy and palaeoenvironmental analysis of a Lower to Middle Jurassic succession on Anholt, Denmark. Journal of Micropalaeontology Vol. 12 # 2 P. 201- 218
- Koppelhus,E.B. et al. (1996) Applicaton of a palynomorph zonation to a series of short borehole sections, Lower to Middle Jurassic, Oresund, Denmark. (Principles and Applications. Applications. J.Jansonius and D.C.McGregor, editors.) American Association of Stratigraphic Palynologists:Principles and Applications. Applications (J.Jansonius and D.C.McGregor,Editors) Vol. 2 P. 779- 793
- Michelsen,O. (1979) Report on the Jurassic of the Hobro No.1 and Voldum No.1 Borings, Denmark. Danmarks Geologiske Undersиgelse P. 141- 149
- Wade-Murphy, J., Kuerschner, W. M., (2006). A new technique to infer the botanical affinity of palynomorphs, and its application on spheripollenites psilatus from the toarcian of bornholm, denmark. In 7 th European Palaeobotany Palynology Conference, Prague (p. 153).
- Wade-Murphy, Kuerschner, W. M., & Hesselbo, S. P. (2006): Abrupt and gradual vegetation changes associated with Toarcian global change inferred from high resolution palynological study of the Korsodde section on Bornholm (dK). In Geophysical Research Abstracts (Vol. 8, p. 00357).
- Möller, H. (1902). Bidrag till Bornholms fossila flora: Pteridofyter (Vol. 38, No. 5). E. Malmströms boktryckeri.
- Mehlqvist, K., Vajda, V., & Larsson, L. M. (2009). A Jurassic (Pliensbachian) flora from Bornholm, Denmark–a study of a historic plant-fossil collection at Lund University, Sweden. GFF, 131(1-2), 137-146.
- McElwain, J. C., Wade-Murphy, J., & Hesselbo, S. P. (2005). Changes in carbon dioxide during an oceanic anoxic event linked to intrusion into Gondwana coals. Nature, 435(7041), 479-482.
- Bromley, R. G., & Uchman, A. (2003). Trace fossils from the Lower and Middle Jurassic marginal marine deposits of the Sorthat Formation, Bornholm, Denmark. Bulletin of the Geological Society of Denmark, 52, 185-208.
- Keighley, D. G., & Pickerill, R. K. (1995). Commentary: the ichnotaxa Palaeophycus and Planolites: historical perspectives and recommendations.
- Bandel, K. (1973). A new name for the ichnogenus Cylindrichnus Bandel, 1967. Journal of Paleontology, 1002-1002.
- Osgood, R. G. (1975). The history of invertebrate ichnology. In The study of trace fossils (pp. 3–12). Springer, Berlin, Heidelberg.
- Hertweck, G., Wehrmann, A., & Liebezeit, G. (2007). Bioturbation structures of polychaetes in modern shallow marine environments and their analogues to Chondrites group traces. Palaeogeography, Palaeoclimatology, Palaeoecology, 245(3-4), 382–389.