Foraminifera
Foraminifera (/fəˌræməˈnɪfərə/; Latin for "hole bearers"; informally called "forams") are members of a phylum or class of amoeboid protists characterized by streaming granular ectoplasm for catching food and other uses; and commonly an external shell (called a "test") of diverse forms and materials. Tests of chitin (found in some simple genera, and Textularia in particular) are believed to be the most primitive type. Most foraminifera are marine, the majority of which live on or within the seafloor sediment (i.e., are benthic), while a smaller number float in the water column at various depths (i.e., are planktonic). Fewer are known from freshwater or brackish conditions, and some very few (nonaquatic) soil species have been identified through molecular analysis of small subunit ribosomal DNA.[2][3]
| Foraminifera | |
|---|---|
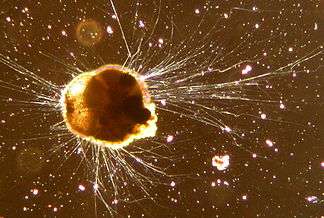 | |
| Live Ammonia tepida (Rotaliida) | |
| Scientific classification | |
| Domain: | |
| (unranked): | |
| (unranked): | |
| Phylum: | |
| Subphylum: | Foraminifera d'Orbigny, 1826 |
| Subdivisions | |
incertae sedis | |
Foraminifera typically produce a test, or shell, which can have either one or multiple chambers, some becoming quite elaborate in structure.[4] These shells are commonly made of calcium carbonate (CaCO
3) or agglutinated sediment particles. Over 50,000 species are recognized, both living (10,000)[5] and fossil (40,000).[6][7] They are usually less than 1 mm in size, but some are much larger, the largest species reaching up to 20 cm.[8]
In modern scientific English, the term foraminifera is both singular and plural (irrespective of the word's Latin derivation), and is used to describe one or more specimens or taxa: its usage as singular or plural must be determined from context. Foraminifera is frequently used informally to describe the group, and in these cases is generally lowercase.[9]
History of study
The earliest known reference to foraminifera comes from Herodotus, who in the 5th century BCE noted them as making up the rock that forms the Great Pyramid of Giza. These are today recognized as representatives of the genus Nummulites. Strabo, in the 1st Century BCE, noted the same foraminifera, and suggested that they were the remains of lentils left by the workers who built the pyramids.[10]
Robert Hooke observed a foraminifera under the microscope, as described and illustrated in his 1665 book Micrographia:
I was trying several small and single Magnifying Glasses, and casually viewing a parcel of white Sand, when I perceiv'd one of the grains exactly shap'd and wreath'd like a Shell[...] I view'd it every way with a better Microscope and found it on both sides, and edge-ways, to resemble the Shell of a small Water-Snail with a flat spiral Shell[...][11]
Antonie van Leeuwenhoek described and illustrated foraminiferal tests in 1700, describing them as minute cockles; his illustration is recognizable as being Elphidium.[12] Early workers classified foraminifera within the genus Nautilus, noting their similarity to certain cephalopods. It was recognised by Lorenz Spengler in 1781 that foraminifera had holes in the septa, which would eventually grant the group its name.[13] Spengler also noted that the septa of foraminifera arced the opposite way from those of nautili and that they lacked a nerve tube.[14]

Alcide d'Orbigny, in his 1826 work, considered them to be a group of minute cephalopods and noted their odd morphology, interpreting the pseudopodia as tentacles and noting the highly reduced (in actuality, absent) head.[15] He named the group foraminifères, or "hole-bearers", as members of the group had holes in the divisions between compartments in their shells, in contrast to nautili or ammonites.[9]
The protozoan nature of foraminifera was first recognized by Dujardin in 1835.[13] Shortly after, in 1852, d'Orbigny produced a classification scheme, recognising 72 genera of foraminifera, which he classified based on test shape—a scheme that drew severe criticism from colleagues.[12]
H.B. Brady's 1884 monograph described the foraminiferal finds of the Challenger expedition. Brady recognized 10 families with 29 subfamilies, with little regard to stratigraphic range; his taxonomy emphasized the idea that multiple different characters must separate taxonomic groups, and as such placed agglutinated and calcareous genera in close relation.
This overall scheme of classification would remain until Cushman's work in the late 1920s. Cushman viewed wall composition as the single most important trait in classification of foraminifera; his classification became widely accepted but also drew criticism from colleagues for being "not biologically sound".
Cushman's scheme nevertheless remained the dominant scheme of classification until Tappan and Loeblich's 1964 classification, which placed foraminifera into the general groupings still used today, based on microstructure of the test wall.[12] These groups have been variously moved around according to different schemes of higher-level classification. Pawlowski's (2013) use of molecular systematics has generally confirmed Tappan and Loeblich's groupings, with some being found as polyphyletic or paraphyletic; this work has also helped to identify higher-level relationships among major foraminiferal groups.[16]
Taxonomy
The taxonomic position of the Foraminifera has varied since Schultze in 1854,[17] who referred to as an order, Foraminiferida. Loeblich and Tappan (1992) reranked Foraminifera as a class[18] as it is now commonly regarded.
The Foraminifera have typically been included in the Protozoa,[19][20][21] or in the similar Protoctista or Protist kingdom.[22][23] Compelling evidence, based primarily on molecular phylogenetics, exists for their belonging to a major group within the Protozoa known as the Rhizaria.[19] Prior to the recognition of evolutionary relationships among the members of the Rhizaria, the Foraminifera were generally grouped with other amoeboids as phylum Rhizopodea (or Sarcodina) in the class Granuloreticulosa.
The Rhizaria are problematic, as they are often called a "supergroup", rather than using an established taxonomic rank such as phylum. Cavalier-Smith defines the Rhizaria as an infra-kingdom within the kingdom Protozoa.[19]
Some taxonomies put the Foraminifera in a phylum of their own, putting them on par with the amoeboid Sarcodina in which they had been placed.
Although as yet unsupported by morphological correlates, molecular data strongly suggest the Foraminifera are closely related to the Cercozoa and Radiolaria, both of which also include amoeboids with complex shells; these three groups make up the Rhizaria.[20] However, the exact relationships of the forams to the other groups and to one another are still not entirely clear. Foraminifera are closely related to testate amoebae.[24]
| Taxonomy from Mikhalevich 2013[25] |
|---|
* Foraminifera d'Orbigny 1826
|
Biology

Modern Foraminifera are primarily marine organisms, but living individuals have been found in brackish, freshwater[26] and even terrestrial habitats.[3] The majority of the species are benthic, and a further 40 morphospecies are planktonic.[27] This count may, however, represent only a fraction of actual diversity, since many genetically distinct species may be morphologically indistinguishable.[28]
A number of forams have unicellular algae as endosymbionts, from diverse lineages such as the green algae, red algae, golden algae, diatoms, and dinoflagellates.[27] These mixotrophic foraminifers are particularly common in nutrient-poor oceanic waters.[29] Some forams are kleptoplastic, retaining chloroplasts from ingested algae to conduct photosynthesis.[30]
The foraminiferal cell is divided into granular endoplasm and transparent ectoplasm from which a pseudopodial net may emerge through a single opening or through many perforations in the test. Individual pseudopods characteristically have small granules streaming in both directions.[26] The pseudopods are used for locomotion, anchoring, excretion, test construction and in capturing food, which consists of small organisms such as diatoms or bacteria.[27][31] Certain foraminifera prey upon small animals such as copepods or cumaceans; some forams even predate upon other forams, drilling holes into the tests of their prey.[32]
Certain benthic foraminifera have been found to be capable of surviving anoxic conditions for over 24 hours, indicating that they are capable of selective anaerobic respiration. This is interpreted as an adaptation to survive changing oxygenic conditions near the sediment-water interface.[33]
Reproduction
The generalized foraminiferal life-cycle involves an alternation between haploid and diploid generations, although they are mostly similar in form.[17][34] The haploid or gamont initially has a single nucleus, and divides to produce numerous gametes, which typically have two flagella. The diploid or agamont is multinucleate, and after meiosis divides to produce new gamonts. Multiple rounds of asexual reproduction between sexual generations are not uncommon in benthic forms.[26]
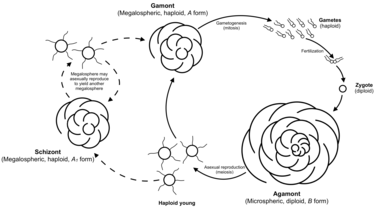
Foraminifera exhibit morphological dimorphism associated with their reproductive cycle. The gamont, or sexually reproducing haploid form, is megalospheric—that is, its proloculus, or first chamber, is proportionally large. The gamont is also known as the A form. Gamonts, despite having typically larger proloculi, also generally have smaller overall test diameter than do agamonts.
After reaching maturity, the gamont divides via mitosis to produce thousands of gametes which are also haploid. These gametes all have a full set of organelles, and are expelled from the test into the environment leaving the test undamaged. Gametes are not differentiated into sperm and egg, and any two gametes from a species can generally fertilize each other.
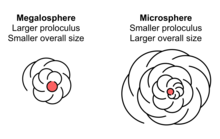
When two gametes combine, they create a diploid, multi-nucleated cell known as the agamont, or B form. In contrast to the gamont, the agamont is microspheric, with a proportionally small first chamber but typically larger overall diameter with more chambers. The agamont is the asexual reproduction phase of the foraminifera; upon reaching adulthood, the protoplasm entirely vacates the test and divides its cytoplasm meiotically via multiple fission to form a number of haploid offspring. These offspring then begin to form their megalospheric first chamber before dispersing.
In some cases the haploid young may mature into a megalospheric form which then reproduces asexually to produce another megalospheric, haploid offspring. In this case, the first megalospheric form is referred to as the schizont or A1 form, while the second is referred to as the gamont or A2 form.

Maturation and reproduction occur more slowly in cooler and deeper water; these conditions also cause forams to grow larger. A forms always seem to be much more numerous than are B forms, likely due to the reduced likelihood of two gametes encountering one another and successfully combining.[35][31]
Variations in reproductive mode
There is a high degree of degree of diversity in reproductive strategies in different foraminiferal groups.
In unilocular species, the A form and B form are still present. As in the microspheric morph of multilocular forams, the asexually reproducing B form is larger than the sexually reproducing A form.
Forams in the family Spirillinidae have amoeboid gametes rather than flagellated. Other aspects of reproduction in this group are generally similar to that of other groups of forams.
The calcareous spirillinid Patellina corrugata has a slightly different reproductive strategy than most other foraminifera. The asexually reproducing B form produces a cyst that surrounds the entire cell; it then divides within this cyst and the juvenile cells cannibalise the calcite of the parent's test to form the first chamber of their own test. These A forms, upon maturity, gather into groups of up to 9 individuals; they then form a protective cyst around the whole group. Gametogenesis occurs within this cyst, producing very low numbers of gametes. The B form larvae are produced inside of the cyst; any nuclei that are not bound into cells are consumed as food for the developing larvae. Patellina in A form is reportedly dioecious, with sexes referred to as the "plus" and "minus"; these sexes differ in number of nuclei, with the "plus" form having three nuclei and the "minus" form having four nuclei. The B form is again larger than the A form.[31][35][32]
Tests
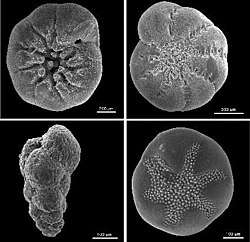
Foraminiferal tests serve to protect the organism within. Owing to their generally hard and durable construction (compared to other protists), the tests of foraminifera are a major source of scientific knowledge about the group. Openings in the test that allow the cytoplasm to extend outside are called apertures.[36] The primary aperture, leading to the exterior, take many different shapes in different species, including but not limited to rounded, crescent-shaped, slit-shaped, hooded, radiate (star-shaped), dendritic (branching). Some foraminifera have "toothed", flanged, or lipped primary apertures. There may be only one primary aperture or multiple; when multiple are present, they may be clustered or equatorial. In addition to the primary aperture, many foraminifera have supplemental apertures. These may form as relict apertures (past primary apertures from an earlier growth stage) or as unique structures.

Test shape is highly variable among different foraminifera; they may be single-chambered (unilocular) or multi-chambered (multilocular). In multilocular forms, new chambers are added as the organism grows. A wide variety of test morphologies is found in both unilocular and multilocular forms, including spiraled, serial, and milioline, among others.[31]
Many foraminifera exhibit dimorphism in their tests, with megalospheric and microspheric individuals. These names should not be taken as referring to the size of the full organism; rather, they refer to the size of the first chamber, or proloculus.
Unlike other shell-secreting organisms, such as molluscs or corals, the tests of foraminifera are located inside the cell membrane, within the protoplasm. The organelles of the cell are located within the compartment(s) of the test, and the hole(s) of the test allow the transfer of material from the pseudopodia to the internal cell and back.[31]
Tests as fossils are known from as far back as the Ediacaran period,[37] and many marine sediments are composed primarily of them. For instance, the limestone that makes up the pyramids of Egypt is composed almost entirely of nummulitic benthic Foraminifera.[38] It is estimated that reef Foraminifera generate about 43 million tons of calcium carbonate per year.[39]
Genetic studies have identified the naked amoeba Reticulomyxa and the peculiar xenophyophores as foraminiferans without tests. A few other amoeboids produce reticulose pseudopods, and were formerly classified with the forams as the Granuloreticulosa, but this is no longer considered a natural group, and most are now placed among the Cercozoa.[40]
Test composition
The form and composition of their tests are the primary means by which forams are identified and classified. Most secrete calcareous tests, composed of calcium carbonate.[26] Calcareous tests may be composed of either aragonite or calcite depending on species; among those with calcite tests, the test may contain either a high or low fraction of magnesium substitution.[12] The test contains an organic matrix, which can sometimes be recovered from fossil samples.[12]
Some studies suggest a high amount of homoplasy in foraminifera, and that neither agglutinated nor calcareous foraminifera form monophyletic groupings.[16]
Soft tests
In some forams, the tests may be composed of organic material, typically the protein tectin. Tectin walls may have sediment particles loosely adhered onto the surface.[31] The foram Reticulomyxa entirely lacks a test, having only a membranous cell wall.[41] Organic-walled forams have traditionally been grouped as the "allogromiids"; however, genetic studies have found that this does not make up a natural group.[16]
Agglutinated tests
Other forams have tests made from small pieces of sediment cemented together (agglutinated) by either proteins (possibly collagen-related), calcium carbonate, or Iron (III) oxide.[31][42] In the past these forms were grouped together as the single-chambered "astrorhizids" and the multi-chambered textulariids. However, recent genetic studies suggest that "astrorhizids" do not make up a natural grouping, instead forming a broad base of the foram tree.[16]

Agglutinating foraminifera may be selective regarding what particles they incorporate into their shells. Some species prefer certain sizes and types of rock particles; other species are preferential towards certain biological materials. Certain species of foraminifera are known to have preferentially agglutinated coccoliths to form their tests; others preferentially utilise echinoderm plates, diatoms, or even other foraminiferans' tests.[43]
The foraminifera Spiculosiphon preferentially agglutinates silica sponge spicules using an organic cement; it shows strong selectivity also towards shape, utilising elongated spicules on its "stalk" and shortened ones on its "bulb". It is thought to use the spicules as both a means of elevating itself off the seabed as well as to lengthen the reach of its pseudopodia to capture prey.[42]
The agglutinated tests of xenophyophores are the largest of any foraminifera, reaching up to 20cm in diameter. The name "xenophyophore", meaning "bearer of foreign bodies", refers to this agglutinating habit. Xenophyophores selectively uptake sediment grains between 63 and 500µm, avoiding larger pebbles and finer silts; type of sediment seems to be a strong factor in which particles are agglutinated, as particle type preferentially includes sulfides, oxides, volcanic glass, and especially tests of smaller foraminifera. Xenophyophores 1.5cm in diameter have been recorded completely naked, with no test whatsoever.[44]
Calcareous tests
Of those foraminifera with calcareous tests, several different structures of calcite crystals are found.

Porcelaneous walls are found in the Miliolida. These consist of high-magnesium calcite organized with an ordered outer and inner calcite lining (the "extrados" and "intrados", respectively) and randomly oriented needle-shaped calcite crystals forming a thick center layer (the "porcelain"). An organic inner lining is also present. The external surface may have a pitted structure, but it is not perforated by holes.[45][46]
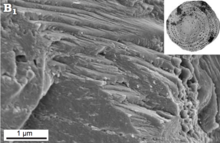
A "monocrystalline" test structure has traditionally been described for the Spirillinida. However, these tests remain poorly understood and poorly described. Some supposed "monocrystalline" spirillinids have been found to actually have tests consisting of a mosaic of very small crystals when observed with scanning electron microscope. SEM observation of Patellina sp. suggests that a truly monocrystalline test may indeed be present, with apparent cleavage faces.[47]
Lagenid tests consist of "fibre bundles" that can reach tens of micrometres long; each "bundle" is formed from a single calcite crystal, is triangular in cross-section, and has a pore in the centre (thought to be an artefact of test deposition). There is also an internal organic layer, attached to the "cone" structure of the fibre bundles. As the crystalline structure varies significantly from that of other calcareous foraminifera, it is thought to represent a separate evolution of the calcareous test. The exact mineralisation process of lagenids remains unclear.[48]
Rotaliid tests are described as "hyaline". They are formed from low-to-high-magnesium calcite "nanograins" positioned with their C-axes perpendicular to the external surface of the test. The test wall is characteristically bilamellar (two-layered) and perforated throughout with small pores. The outer calcite layer of the test wall is referred to as the "outer lamina" while the inner calcite layer is referred to as the "inner lining"; this should not be confused with the organic inner lining beneath the test. Sandwiched between the outer lamina and the inner lining is the "median layer", a protein layer that separates the two. The median layer is quite variable; depending on the species it may be well-defined while in others it is not sharply delineated. Some genera may contain sediment particles within the median layer.[31][49][48]
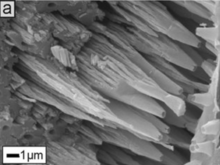
The Carterinids, including the genera Carterina and Zaninettia, have a unique crystalline structure of the test which long complicated their classification. The test in this genus consists of spicules of low-magnesium calcite, bound together with an organic matrix and containing "blebs" of organic matter; this led some researchers to conclude that the test must be agglutinated. However, life studies have failed to find agglutination, and in fact the genus has been discovered on artificial substrate where sediment particles do not accumulate.[50] A 2014 genetic study found carterinids to be an independent lineage within the Globothalamea, and supported the idea of the spicules being secreted as spicule shape differed consistently between specimens of Carterina and Zaninettia collected from the same locality (ovoid in Carterina, rounded-rectangular in Zaninettia).[51]
The now-extinct Fusulinids have traditionally been considered unique in having tests of homogenous microgranular crystals with no preferred orientation and almost no cement. However, a 2017 study found that the supposed microgranular structure was actually the result of diagenetic alteration of the fossils, and that unaltered fusulinid tests instead had a hyaline structure. This suggests that the group is affiliated with the Globothalamea.[47]
Robertinids have aragonitic tests with perforations; these are similar to the tests of rotaliids in that they are formed from nanograins, however, they differ in composition and in having well-organised columnar domains. As the earliest planktonic forams had aragonitic tests, it has been suggested that this may represent a separate evolution of a planktonic lifestyle within the Robertinida, rather than being close relatives of Globigerinans.[46]
Hyaline aragonitic tests are also present in the Involutinida.[48]
Silica tests
One genus, Miliamellus, has a non-perforated test made of opaline silica.[18]
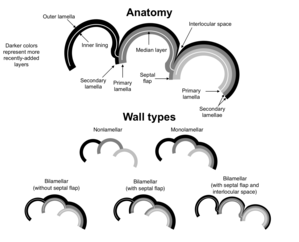
Test wall construction
When a secreted test is present, walls of foraminiferal tests may be either nonlamellar or lamellar.
Nonlamellar walls are found in some foraminifera, such as Carterinida, Spirillinida, and Miliolida. In these forms, the secretion of a new chamber is not associated with any further deposition over previous chambers. As such there is no associated layering of calcite layers on the test.[49]
In foraminifera with lamellar walls, the deposition of a new chamber is accompanied by the deposition of a layer over previously-formed chambers. This layer may cover all previous chambers, or it may cover only some of them. These layers are known as secondary lamellae.
Foraminifera with lamellar walls can be further broken down into those with monolamellar walls and those with bilamellar walls. Monolamellar foraminifera secrete test walls which consist of a single layer, while those of bilamellar foraminifera are double-layered with an organic "median layer", sometimes containing sediment particles. In the case of bilamellar foraminifera, the outer layer is referred to as the "outer lamella" whilst the inner layer is referred to as the "inner lining". Monolamellar forams include the Lagenida, while bilamellar forms include the Rotaliida (including the planktonic subgroup, the Globigerinina).[49]
Bilamellar test walls can be further divided into those with septal flaps (a layer of test wall covering the previously-secreted septum) and those lacking septal flaps. Septal flaps are not known to be present in any foraminifera other than those with bilamellar walls.
The presence of a septal flap is often, though not always, associated with the presence of an interlocular space. As the name suggests, this is a small space located between chambers; it may be open and form part of the outer surface of the test, or it may be enclosed to form a void. The layer enclosing the void is formed from different parts of the lamellae in different genera, suggesting an independent evolution of enclosed interlocular spaces in order to strengthen the test.[49]
Deep-sea species
Foraminifera are found in the deepest parts of the ocean such as the Mariana Trench, including the Challenger Deep, the deepest part known. At these depths, below the carbonate compensation depth, the calcium carbonate of the tests is soluble in water due to the extreme pressure. The Foraminifera found in the Challenger Deep thus have no carbonate test, but instead have one of organic material.[52]
Four species found in the Challenger Deep are unknown from any other place in the oceans, one of which is representative of an endemic genus unique to the region. They are Resigella laevis and R. bilocularis, Nodellum aculeata, and Conicotheca nigrans (the unique genus). All have tests that are mainly of transparent organic material which have small (about 100 nm) plates that appear to be clay.[52]
Evolutionary history
Molecular clocks indicate that the crown-group of foraminifera likely evolved during the Neoproterozoic, between 900 and 650 million years ago; this timing is consistent with Neoproterozoic fossils of the closely related filose amoebae. As fossils of foraminifera have not been found prior to the very end of the Ediacaran, it is likely that most of these Proterozoic forms did not have hard-shelled tests.[53][54]
Due to their non-mineralised tests, "allogromiids" have no fossil record.[53]
The mysterious vendozoans of the Ediacaran period have been suggested to represent fossil xenophyophores.[55] However, the discovery of diagenetically-altered C27 sterols associated with the remains of Dickinsonia cast doubt on this identification and suggest it may instead be an animal.[56] Other researchers have suggested that the elusive trace fossil Paleodictyon and its relatives may represent a fossil xenophyophore[57] and noted the similarity of the extant xenophyophore Occultammina to the fossil;[58] however, modern examples of Paleodictyon have not been able to clear up the issue and the trace may alternately represent a burrow or a glass sponge.[59] Supporting this notion is the similar habitat of living xenophyophores to the inferred habitat of fossil graphoglyptids; however, the large size and regularity of many graphoglyptids as well as the apparent absence of xenophyae in their fossils casts doubt on the possibility.[58] As of 2017 no definite xenophyophore fossils have been found.[60]
Test-bearing foraminifera have an excellent fossil record throughout the Phanerozoic eon. The earliest known definite foraminifera appear in the fossil record towards the very end of the Ediacaran; these forms all have agglutinated tests and are unilocular. These include forms like Platysolenites and Spirosolenites.[61][37]
Single-chambered foraminifera continued to diversity throughout the Cambrian. Some commonly encountered forms include Ammodiscus, Glomospira, Psammosphera, and Turritellella; these species are all agglutinated. They make up part of the Ammodiscina, a lineage of spirillinids that still contains modern forms.[62][16] Later spirillinids would evolve multilocularity and calcitic tests, with the first such forms appearing during the Triassic; the group saw little effects on diversity due to the K-Pg extinction.[63]
The earliest multi-chambered foraminifera are agglutinated species, and appear in the fossil record during the middle Cambrian period. Due to their poor preservation they cannot be positively assigned to any major foram group.[62]

The earliest known calcareous-walled foraminifera are the Fusulinids, which appear in the fossil record during the Llandoverian epoch of the early Silurian. The earliest of these were microscopic, planispirally coiled, and evolute; later forms evolved a diversity of shapes including lenticular, globular, and perhaps most famously, elongated rice-shaped forms. Later species of fusulinids grew to much larger size, with some forms reaching 5 cm in length; reportedly, some specimens reach up to 14 cm in length, making them among the largest foraminifera extant or extinct. Fusulinids are the earliest lineage of foraminifera thought to have evolved symbiosis with photosynthetic organisms. Fossils of fusulinids have been found on all continents except Antarctica; they reached their greatest diversity during the Visean epoch of the Carboniferous. The group then gradually declined in diversity until finally going extinct during the Permo-Triassic extinction event.[31][63][64]
During the Tournaisian epoch of the Carboniferous, Miliolid foraminifera first appeared in the fossil record, having diverged from the spirillinids within the Tubothalamea. Miliolids suffered about 50% casualties during both the Permo-Triassic and K-Pg extinctions but survived to the present day. Some fossil miliolids reached up to 2 cm in diameter.[63]
The earliest known Lagenid fossils appear during the Moscovian epoch of the Carboniferous. Seeing little effect due to the Permo-Triassic or K-Pg extinctions, the group diversified through time. Secondarily unilocular taxa evolved during the Jurassic and Cretaceous.
The earliest Involutinid fossils appear during the Permian; the lineage diversified throughout the Mesozoic of Eurasia before apparently vanishing from the fossil record following the Cenomanian-Turonian Ocean Anoxic Event. The extant group planispirillinidae has been referred to the involutinida, but this remains the subject of debate.[65][63]
The Robertinida first appear in the fossil record during the Anisian epoch of the Triassic. The group remained at low diversity throughout its fossil history; all living representatives belong to the Robertinidae, which first appeared during the Paleocene.[63]
The first definite Rotaliid fossils do not appear in the fossil record until the Pliensbachian epoch of the Jurassic, following the Triassic-Jurassic event.[66] Diversity of the group remained low until the aftermath of the Cenomanian-Turonian event, after which the group saw a rapid diversification. Of this group, the planktonic Globigerinina first appear in the aftermath of the Toarcian Turnover; the group saw heavy losses during both the K-Pg extinction and the Eocene-Oligocene extinction, but remains extant and diverse to this day.[63]
Paleontological applications


Dying planktonic Foraminifera continuously rain down on the sea floor in vast numbers, their mineralized tests preserved as fossils in the accumulating sediment. Beginning in the 1960s, and largely under the auspices of the Deep Sea Drilling, Ocean Drilling, and International Ocean Drilling Programmes, as well as for the purposes of oil exploration, advanced deep-sea drilling techniques have been bringing up sediment cores bearing Foraminifera fossils.[67] The effectively unlimited supply of these fossil tests and the relatively high-precision age-control models available for cores has produced an exceptionally high-quality planktonic Foraminifera fossil record dating back to the mid-Jurassic, and presents an unparalleled record for scientists testing and documenting the evolutionary process.[67] The exceptional quality of the fossil record has allowed an impressively detailed picture of species inter-relationships to be developed on the basis of fossils, in many cases subsequently validated independently through molecular genetic studies on extant specimens[68]
Because certain types of foraminifera are found only in certain environments, their fossils can be used to figure out the kind of environment under which ancient marine sediments were deposited; conditions such as salinity, depth, oxygenic conditions, and light conditions can be determined from the different habitat preferences of various species of forams. This allows workers to track changing climates and environmental conditions over time by aggregating information about the foraminifera present.[69]
In other cases, the relative proportion of planktonic to benthic foraminifera fossils found in a rock can be used as a proxy for the depth of a given locality when the rocks were being deposited.[70]
Foraminifera have significant application in the field of biostratigraphy. Due to their small size and hard shells, foraminifera may be preserved in great abundance and with high quality of preservation; due to their complex morphology, individual species are easily recognizable. Foraminifera species in the fossil record have limited ranges between the species' first evolution and their disappearance; stratigraphers have worked out the successive changes in foram assemblages throughout much of the Phanerozoic. As such, the assemblage of foraminifera within a given locality can be analyzed and compared to known dates of appearance and disappearance in order to narrow down the age of the rocks. This allows paleontologists to interpret the age of sedimentary rocks when radiometric dating is not applicable.[71] This application of foraminifera was discovered by Alva C. Ellisor in 1920.[72]
Calcareous fossil foraminifera are formed from elements found in the ancient seas where they lived. Thus, they are very useful in paleoclimatology and paleoceanography. They can be used, as a climate proxy, to reconstruct past climate by examining the stable isotope ratios and trace element content of the shells (tests). Global temperature and ice volume can be revealed by the isotopes of oxygen, and the history of the carbon cycle and oceanic productivity by examining the stable isotope ratios of carbon;[73] see δ18O and δ13C. The concentration of trace elements, like magnesium (Mg),[74] lithium (Li)[75] and boron (B),[76] also hold a wealth of information about global temperature cycles, continental weathering, and the role of the ocean in the global carbon cycle. Geographic patterns seen in the fossil records of planktonic forams are also used to reconstruct ancient ocean currents.
Modern uses
The oil industry relies heavily on microfossils such as forams to find potential hydrocarbon deposits.[77]
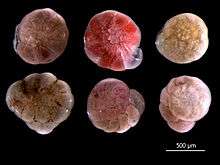
For the same reasons they make useful biostratigraphic markers, living foraminiferal assemblages have been used as bioindicators in coastal environments, including indicators of coral reef health. Because calcium carbonate is susceptible to dissolution in acidic conditions, foraminifera may be particularly affected by changing climate and ocean acidification.

Foraminifera have many uses in petroleum exploration and are used routinely to interpret the ages and paleoenvironments of sedimentary strata in oil wells.[78] Agglutinated fossil foraminifera buried deeply in sedimentary basins can be used to estimate thermal maturity, which is a key factor for petroleum generation. The Foraminiferal Colouration Index[79] (FCI) is used to quantify colour changes and estimate burial temperature. FCI data is particularly useful in the early stages of petroleum generation (about 100 °C).
Foraminifera can also be used in archaeology in the provenancing of some stone raw material types. Some stone types, such as limestone, are commonly found to contain fossilised foraminifera. The types and concentrations of these fossils within a sample of stone can be used to match that sample to a source known to contain the same "fossil signature".[80]
Gallery
 Foraminifera of Pag Island, Adriatic Sea -60 m, field width 5.5 mm
Foraminifera of Pag Island, Adriatic Sea -60 m, field width 5.5 mm Foraminifera of Pag Island, Adriatic Sea -60 m, field width 5.5 mm
Foraminifera of Pag Island, Adriatic Sea -60 m, field width 5.5 mm Foraminifera of Pag Island, Adriatic Sea -60 m, field width 5.5 mm
Foraminifera of Pag Island, Adriatic Sea -60 m, field width 5.5 mm Foraminifera of Pag Island, Adriatic Sea -60 m, field width 5.5 mm
Foraminifera of Pag Island, Adriatic Sea -60 m, field width 5.5 mm Foraminifera of Indian Ocean, south-eastern coast of Bali, field width 5.5 mm
Foraminifera of Indian Ocean, south-eastern coast of Bali, field width 5.5 mm Foraminifera of Indian Ocean, south-eastern coast of Bali, field width 5.5 mm
Foraminifera of Indian Ocean, south-eastern coast of Bali, field width 5.5 mm Foraminifera of Indian Ocean, south-eastern coast of Bali, field width 5.5 mm
Foraminifera of Indian Ocean, south-eastern coast of Bali, field width 5.5 mm Foraminifera in Ngapali, Myanmar, field width 5.22 mm
Foraminifera in Ngapali, Myanmar, field width 5.22 mm Foraminifera Heterostegina depressa, field width 4.4 mm
Foraminifera Heterostegina depressa, field width 4.4 mm
References
- Parfrey, Laura Wegener; Lahr, Daniel J. G.; Knoll, Andrew H.; Katz, Laura A. (16 August 2011). "Estimating the timing of early eukaryotic diversification with multigene molecular clocks". Proceedings of the National Academy of Sciences of the United States of America. 108 (33): 13624–13629. Bibcode:2011PNAS..10813624P. doi:10.1073/pnas.1110633108. PMC 3158185. PMID 21810989.
- Giere, Olav (2009). Meiobenthology: the microscopic motile fauna of aquatic sediments (2nd ed.). Berlin: Springer. ISBN 978-3540686576.
- Lejzerowicz, Franck; Pawlowski, Jan; Fraissinet-Tachet, Laurence; Marmeisse, Roland (1 September 2010). "Molecular evidence for widespread occurrence of Foraminifera in soils". Environmental Microbiology. 12 (9): 2518–26. doi:10.1111/j.1462-2920.2010.02225.x. PMID 20406290.
- Kennett, J.P.; Srinivasan, M.S. (1983). Neogene planktonic foraminifera: a phylogenetic atlas. Hutchinson Ross. ISBN 978-0-87933-070-5.
- Ald, S.M. et al. (2007) Diversity, Nomenclature, and Taxonomy of Protists, Syst. Biol. 56(4), 684–689, DOI: 10.1080/10635150701494127.
- Pawlowski, J., Lejzerowicz, F., & Esling, P. (2014). Next-generation environmental diversity surveys of foraminifera: preparing the future. The Biological Bulletin, 227(2), 93-106.
- "World Foraminifera Database".
- Marshall M (3 February 2010). "Zoologger: 'Living beach ball' is giant single cell". New Scientist.
- Lipps JH, Finger KL, Walker SE (October 2011). "What Should We call the Foraminifera" (PDF). Journal of Foraminiferal Research. 41 (4): 309–313. doi:10.2113/gsjfr.41.4.309. Retrieved 10 April 2018.
- "Foraminifera | Fossil Focus | Time | Discovering Geology | British Geological Survey (BGS)". www.bgs.ac.uk. Retrieved 20 July 2020.
- "Micrographia, or, Some physiological descriptions of minute bodies made by magnifying glasses ?with observations and inquiries thereupon /by R. Hooke ... : Hooke, Robert, : Free Download, Borrow, and Streaming". Internet Archive. Retrieved 20 July 2020.
- Sen Gupta, Barun K. (2003), Sen Gupta, Barun K. (ed.), Modern Foraminifera, Springer Netherlands, pp. 7–36, doi:10.1007/0-306-48104-9_2, ISBN 978-0-306-48104-8 Missing or empty
|title=(help);|chapter=ignored (help) - BOUDAGHER-FADEL, MARCELLE K. (2018), "Biology and Evolutionary History of Larger Benthic Foraminifera", Evolution and Geological Significance of Larger Benthic Foraminifera (2 ed.), UCL Press, pp. 1–44, ISBN 978-1-911576-94-5, retrieved 19 July 2020
- Hansen, H. (1 January 1981). "On Lorentz Spengler and a neotype for the foraminifer Calcarina spengleri". Cite journal requires
|journal=(help) - d'Orbigny, Alcide (1826). "Tableau Méthodique de la Classe des Céphalopodes". Annales des Sciences Naturelles, Paris (Série 1). 7: 245–314 – via Biodiversity Heritage Library.
- Pawlowski, Jan; Holzmann, Maria; Tyszka, Jarosław (1 April 2013). "New supraordinal classification of Foraminifera: Molecules meet morphology". Marine Micropaleontology. 100: 1–10. doi:10.1016/j.marmicro.2013.04.002. ISSN 0377-8398.
- Loeblich Jr, A.R.; Tappan, H. (1964). "Foraminiferida". Part C, Protista 2. Treatise on Invertebrate Paleontology. Geological Society of America. pp. C55–C786. ISBN 978-0-8137-3003-5.
- Sen Gupta, Barun K. (2002). Modern Foraminifera. Springer. p. 16. ISBN 978-1-4020-0598-5.
- Cavalier-Smith, T (2004). "Only Six Kingdoms of Life" (PDF). Proceedings. Biological Sciences. 271 (1545): 1251–62. doi:10.1098/rspb.2004.2705. PMC 1691724. PMID 15306349.
- Cavalier-Smith, T (2003). "Protist phylogeny and the high-level classification of Protozoa". European Journal of Protistology. 34 (4): 338–348. doi:10.1078/0932-4739-00002.
- Tolweb Cercozoa
- European Register of Marine Species
- eForams-taxonomy Archived 3 October 2011 at the Wayback Machine
- Testate amoebae as environmental indicators (PDF), archived from the original (PDF) on 27 November 2016, retrieved 27 November 2016
- Mikhalevich, V.I. (2013). "New insight into the systematics and evolution of the foraminifera". Micropaleontology. 59 (6): 493–527.
- Sen Gupta, Barun K. (1982). "Ecology of benthic Foraminifera". In Broadhead, T.W. (ed.). Foraminifera: notes for a short course organized by M.A. Buzas and B.K. Sen Gupta. Studies in Geology. 6. University of Tennessee, Dept. of Geological Sciences. pp. 37–50. ISBN 978-0910249058. OCLC 9276403.
- Hemleben, C.; Anderson, O.R.; Spindler, M. (1989). Modern Planktonic Foraminifera. Springer-Verlag. ISBN 978-3-540-96815-3.
- Kucera, M.; Darling, K.F. (April 2002). "Cryptic species of planktonic foraminifera: their effect on palaeoceanographic reconstructions". Philos Trans Royal Soc A. 360 (1793): 695–718. Bibcode:2002RSPTA.360..695K. doi:10.1098/rsta.2001.0962. PMID 12804300.
- Advances in Microbial Ecology, Volum 11
- Bernhard, J. M.; Bowser, S.M. (1999). "Benthic Foraminifera of dysoxic sediments: chloroplast sequestration and functional morphology". Earth-Science Reviews. 46 (1): 149–165. Bibcode:1999ESRv...46..149B. doi:10.1016/S0012-8252(99)00017-3.
- Saraswati, Pratul Kumar; Srinivasan, M. S. (2016), Saraswati, Pratul Kumar; Srinivasan, M.S. (eds.), "Calcareous-Walled Microfossils", Micropaleontology: Principles and Applications, Springer International Publishing, pp. 81–119, doi:10.1007/978-3-319-14574-7_6, ISBN 978-3-319-14574-7
- Goldstein, Susan T. (2003), Sen Gupta, Barun K. (ed.), Modern Foraminifera, Springer Netherlands, pp. 37–55, doi:10.1007/0-306-48104-9_3, ISBN 978-0-306-48104-8 Missing or empty
|title=(help);|chapter=ignored (help) - Moodley, L.; Hess, C. (1 August 1992). "Tolerance of Infaunal Benthic Foraminifera for Low and High Oxygen Concentrations". The Biological Bulletin. 183 (1): 94–98. doi:10.2307/1542410. ISSN 0006-3185. JSTOR 1542410. PMID 29304574.
- Moore, R.C.; Lalicker, A.G.; Fischer, C.G. (1952). "Ch 2 Foraminifera and Radiolaria". Invertebrate Fossils. McGraw-Hill. OCLC 547380.
- Haynes, J. R. (18 June 1981). Foraminifera. Springer. ISBN 978-1-349-05397-1.
- Lana, C (2001). "Cretaceous Carterina (Foraminifera)". Marine Micropaleontology. 41 (1–2): 97–102. Bibcode:2001MarMP..41...97L. doi:10.1016/S0377-8398(00)00050-5.
- Kontorovich, A. E.; Varlamov, A. I.; Grazhdankin, D. V.; Karlova, G. A.; Klets, A. G.; Kontorovich, V. A.; Saraev, S. V.; Terleev, A. A.; Belyaev, S. Yu.; Varaksina, I. V.; Efimov, A. S. (1 December 2008). "A section of Vendian in the east of West Siberian Plate (based on data from the Borehole Vostok 3)". Russian Geology and Geophysics. 49 (12): 932–939. doi:10.1016/j.rgg.2008.06.012. ISSN 1068-7971.
- Foraminifera: History of Study, University College London, retrieved 20 September 2007
- Langer, M. R.; Silk, M. T. B.; Lipps, J. H. (1997). "Global ocean carbonate and carbon dioxide production: The role of reef Foraminifera". Journal of Foraminiferal Research. 27 (4): 271–277. doi:10.2113/gsjfr.27.4.271.
- Adl, S. M.; Simpson, A. G. B.; Farmer, M. A.; Anderson; et al. (2005). "The new higher level classification of Eukaryotes with emphasis on the taxonomy of Protists". Journal of Eukaryotic Microbiology. 52 (5): 399–451. doi:10.1111/j.1550-7408.2005.00053.x. PMID 16248873.
- Pawlowski, Jan; Bolivar, Ignacio; Fahrni, Jose F.; Vargas, Colomban De; Bowser, Samuel S. (1999). "Molecular Evidence That Reticulomyxa Filosa Is A Freshwater Naked Foraminifer". Journal of Eukaryotic Microbiology. 46 (6): 612–617. doi:10.1111/j.1550-7408.1999.tb05137.x. ISSN 1550-7408.
- Maldonado, Manuel; López-Acosta, María; Sitjà, Cèlia; Aguilar, Ricardo; García, Silvia; Vacelet, Jean (10 June 2013). "A giant foraminifer that converges to the feeding strategy of carnivorous sponges: Spiculosiphon oceana sp. nov. (Foraminifera, Astrorhizida)". Zootaxa. 3669 (4): 571–584. doi:10.11646/zootaxa.3669.4.9. ISSN 1175-5334.
- Thomsen, Erik; Rasmussen, Tine L. (1 July 2008). "COCCOLITH-AGGLUTINATING FORAMINIFERA FROM THE EARLY CRETACEOUS AND HOW THEY CONSTRUCTED THEIR TESTS". Journal of Foraminiferal Research. 38 (3): 193–214. doi:10.2113/gsjfr.38.3.193. ISSN 0096-1191.
- Levin, Lisa A.; Thomas, Cynthia L. (1 December 1988). "The ecology of xenophyophores (Protista) on eastern Pacific seamounts". Deep Sea Research Part A. Oceanographic Research Papers. 35 (12): 2003–2027. doi:10.1016/0198-0149(88)90122-7. ISSN 0198-0149.
- Jain, Sreepat (2020), Jain, Sreepat (ed.), "Benthic Foraminifera", Fundamentals of Invertebrate Palaeontology: Microfossils, Springer Geology, New Delhi: Springer India, pp. 171–192, doi:10.1007/978-81-322-3962-8_9, ISBN 978-81-322-3962-8, retrieved 19 July 2020
- "Chamber arrangement versus wall structure in the high-rank phylogenetic classification of Foraminifera - Acta Palaeontologica Polonica". www.app.pan.pl. Retrieved 19 July 2020.
- Dubicka, Zofia; Gorzelak, Przemysław (9 November 2017). "Unlocking the biomineralization style and affinity of Paleozoic fusulinid foraminifera". Scientific Reports. 7 (1): 1–6. doi:10.1038/s41598-017-15666-1. ISSN 2045-2322.
- Dubicka, Zofia; Owocki, Krzysztof; Gloc, Michał (1 April 2018). "Micro- and Nanostructures of Calcareous Foraminiferal Tests: Insight from Representatives of Miliolida, Rotaliida and Lagenida". Journal of Foraminiferal Research. 48 (2): 142–155. doi:10.2113/gsjfr.48.2.142. ISSN 0096-1191.
- Hansen, Hans Jørgen (2003), Sen Gupta, Barun K. (ed.), "Shell construction in modern calcareous Foraminifera", Modern Foraminifera, Dordrecht: Springer Netherlands, pp. 57–70, doi:10.1007/0-306-48104-9_4, ISBN 978-0-306-48104-8, retrieved 19 July 2020
- Machado, Altair; Barros, Facelucia (8 January 2013). "The occurrence of Carterina spiculotesta (Carter, 1877) on an artificial substrate". Check List. 9(4): 813–814. doi:10.15560/9.4.813. ISSN 1809-127X.
- Pawlowski, Jan; Holzmann, Maria; Debenay, Jean-Pierre (1 October 2014). "MOLECULAR PHYLOGENY OF CARTERINA SPICULOTESTA AND RELATED SPECIES FROM NEW CALEDONIA". Journal of Foraminiferal Research. 44 (4): 440–450. doi:10.2113/gsjfr.44.4.440. ISSN 0096-1191.
- Gooday, A.J.; Todo, Y.; Uematsu, K.; Kitazato, H. (July 2008). "New organic-walled Foraminifera (Protista) from the ocean's deepest point, the Challenger Deep (western Pacific Ocean)". Zoological Journal of the Linnean Society. 153 (3): 399–423. doi:10.1111/j.1096-3642.2008.00393.x.
- Pawlowski, Jan; Holzmann, Maria; Berney, Cédric; Fahrni, José; Gooday, Andrew J.; Cedhagen, Tomas; Habura, Andrea; Bowser, Samuel S. (30 September 2003). "The evolution of early Foraminifera". Proceedings of the National Academy of Sciences. 100 (20): 11494–11498. doi:10.1073/pnas.2035132100. ISSN 0027-8424. PMC 208786. PMID 14504394.
- Groussin, Mathieu; Pawlowski, Jan; Yang, Ziheng (1 October 2011). "Bayesian relaxed clock estimation of divergence times in foraminifera". Molecular Phylogenetics and Evolution. 61 (1): 157–166. doi:10.1016/j.ympev.2011.06.008. ISSN 1055-7903. PMID 21723398.
- Seilacher, A. (1 January 2007). "The nature of vendobionts". Geological Society, London, Special Publications. 286 (1): 387–397. doi:10.1144/SP286.28. ISSN 0305-8719.
- Bobrovskiy, Ilya; Hope, Janet M.; Ivantsov, Andrey; Nettersheim, Benjamin J.; Hallmann, Christian; Brocks, Jochen J. (21 September 2018). "Ancient steroids establish the Ediacaran fossil Dickinsonia as one of the earliest animals". Science. 361 (6408): 1246–1249. doi:10.1126/science.aat7228. ISSN 0036-8075. PMID 30237355.
- Swinbanks, D. D. (1 October 1982). "Piaeodicton: The Traces of Infaunal Xenophyophores?". Science. 218 (4567): 47–49. doi:10.1126/science.218.4567.47. ISSN 0036-8075. PMID 17776707.
- Levin, Lisa A. (1994). "Paleoecology and Ecology of Xenophyophores". PALAIOS. 9 (1): 32–41. doi:10.2307/3515076. ISSN 0883-1351. JSTOR 3515076.
- Rona, Peter A.; Seilacher, Adolf; de Vargas, Colomban; Gooday, Andrew J.; Bernhard, Joan M.; Bowser, Sam; Vetriani, Costantino; Wirsen, Carl O.; Mullineaux, Lauren; Sherrell, Robert; Frederick Grassle, J. (1 September 2009). "Paleodictyon nodosum: A living fossil on the deep-sea floor". Deep Sea Research Part II: Topical Studies in Oceanography. Marine Benthic Ecology and Biodiversity: A Compilation of Recent Advances in Honor of J. Frederick Grassle. 56 (19): 1700–1712. doi:10.1016/j.dsr2.2009.05.015. ISSN 0967-0645.
- Gooday, Andrew J; Holzmann, Maria; Caulle, Clémence; Goineau, Aurélie; Kamenskaya, Olga; Weber, Alexandra A. -T.; Pawlowski, Jan (1 March 2017). "Giant protists (xenophyophores, Foraminifera) are exceptionally diverse in parts of the abyssal eastern Pacific licensed for polymetallic nodule exploration". Biological Conservation. 207: 106–116. doi:10.1016/j.biocon.2017.01.006. ISSN 0006-3207.
- McIlroy, Duncan; Green, O. R.; Brasier, M. D. (2001). "Palaeobiology and evolution of the earliest agglutinated Foraminifera: Platysolenites, Spirosolenites and related forms". Lethaia. 34 (1): 13–29. doi:10.1080/002411601300068170. ISSN 1502-3931.
- Scott, David B.; Medioli, Franco; Braund, Regan (1 June 2003). "Foraminifera from the Cambrian of Nova Scotia: The oldest multichambered foraminifera". Micropaleontology. 49 (2): 109–126. doi:10.2113/49.2.109. ISSN 1937-2795.
- Tappan, Helen; Loeblich, Alfred R. (1988). "Foraminiferal Evolution, Diversification, and Extinction". Journal of Paleontology. 62 (5): 695–714. ISSN 0022-3360. JSTOR 1305391.
- "Fusulinids | GeoKansas". geokansas.ku.edu. Retrieved 16 May 2020.
- Czaplewski, John J. "PBDB Navigator". paleobiodb.org. Retrieved 16 May 2020.
- Gräfe, K.U. (2005). "Benthic foraminifers and palaeoenvironment in the Lower and Middle Jurassic of the Western Basque-Cantabrian Basin (Northern Spain)". Journal of Iberian Geology. 31 (2): 217–233. S2CID 55664447.
- "Nature debates".
- Journal bioinformatics and biology insights, Using the Multiple Analysis Approach to Reconstruct Phylogenetic Relationships among Planktonic Foraminifera from Highly Divergent and Length-polymorphic SSU rDNA Sequences
- Gebhardt, Holger (1 February 1997). "Cenomanian to Turonian foraminifera from Ashaka (NE Nigeria): quantitative analysis and palaeoenvironmental interpretation". Cretaceous Research. 18 (1): 17–36. doi:10.1006/cres.1996.0047. ISSN 0195-6671.
- Báldi, Katalin; Benkovics, László; Sztanó, Orsolya (1 May 2002). "Badenian (Middle Miocene) basin development in SW Hungary: subsidence history based on quantitative paleobathymetry of foraminifera". International Journal of Earth Sciences. 91 (3): 490–504. doi:10.1007/s005310100226. ISSN 1437-3262.
- Australia, c\=AU\;o\=Australia Government\;ou\=Geoscience (15 May 2014). "Biostratigraphy". www.ga.gov.au. Retrieved 20 July 2020.
- Cushman, Joseph A.; Ellisor, Alva C. (1 January 1945). "The Foraminiferal Fauna of the Anahuac Formation". Journal of Paleontology. 19 (6): 545–572. JSTOR 1299203.
- Zachos, J.C.; Pagani, M.; Sloan, L.; Thomas, E.; Billups, K. (2001). "Trends, Rhythms, and Aberrations in Global Climate, 65 Ma to Present". Science. 292 (5517): 686–693. Bibcode:2001Sci...292..686Z. doi:10.1126/science.1059412. PMID 11326091.
- Branson, Oscar; Redfern, Simon A.T.; Tyliszczak, Tolek; Sadekov, Aleksey; Langer, Gerald; Kimoto, Katsunori; Elderfield, Henry (December 2013). "The coordination of Mg in foraminiferal calcite". Earth and Planetary Science Letters. 383: 134–141. Bibcode:2013E&PSL.383..134B. doi:10.1016/j.epsl.2013.09.037.
- Misra, S.; Froelich, P. N. (26 January 2012). "Lithium Isotope History of Cenozoic Seawater: Changes in Silicate Weathering and Reverse Weathering". Science. 335 (6070): 818–823. Bibcode:2012Sci...335..818M. doi:10.1126/science.1214697. PMID 22282473.
- Hemming, N.G.; Hanson, G.N. (January 1992). "Boron isotopic composition and concentration in modern marine carbonates". Geochimica et Cosmochimica Acta. 56 (1): 537–543. Bibcode:1992GeCoA..56..537H. doi:10.1016/0016-7037(92)90151-8.
- Boardman, R.S.; Cheetham, A.H.; Rowell, A.J. (1987). Fossil Invertebrates. Wiley. ISBN 978-0865423022.
- Jones, R.W. (1996). Micropalaeontology in petroleum exploration. Clarendon Press. ISBN 978-0-19-854091-5.
- McNeil, D.H.; Issler, D.R.; Snowdon, L.R. (1996). Colour Alteration, Thermal Maturity, and Burial Diagenesis in Fossil Foraminifers. Geological Survey of Canada Bulletin. 499. Geological Survey of Canada. ISBN 978-0-660-16451-9.
- Wilkinson, Ian P.; Williams, Mark; Young, Jeremy R.; Cook, Samantha R.; Fulford, Michael G.; Lott, Graham K. (1 August 2008). "The application of microfossils in assessing the provenance of chalk used in the manufacture of Roman mosaics at Silchester". Journal of Archaeological Science. 35 (8): 2415–2422. doi:10.1016/j.jas.2008.03.010. ISSN 0305-4403.
External links
| Wikispecies has information related to Foraminifera |
| Wikimedia Commons has media related to Foraminifera. |
- General information
- The University of California Museum of Paleontology website has an Introduction to the Foraminifera
- Researchers at the University of South Florida developed a system using Foraminifera for monitoring coral reef environments
- University College London's micropaleontology site has an overview of Foraminifera, including many high-quality SEMs
- Illustrated glossary of terms used in foraminiferal research is the Lukas Hottinger's glossary published in the OA e-journal "Carnets de Géologie — Notebooks on Geology"
- Information on Foraminifera Martin Langer's Micropaleontology Page
- Benthic Foraminifera information from the 2005 Urbino Summer School of Paleoclimatology
- Online flip-books
- Illustrated glossary of terms used in foraminiferal research by Lukas Hottinger (alternative version of the one published in "Carnets de Géologie — Notebooks on Geology")
- Resources
- pforams@mikrotax - an online database detailing the taxonomy of planktonic foraminifera
- The star*sand project (part of micro*scope) is a cooperative database of information about Foraminifera
- 3D models of forams, generated by X-ray tomography
- CHRONOS has several Foraminifera resources, including a taxon search page and a micro-paleo section NB Most of this content is now included in the pforams@mikrotax website
- eForams is a web site focused on Foraminifera and modeling of foraminiferal shells
- Foraminifera Gallery Illustrated catalog of recent and fossil Foraminifera by genus and locality
- "Foraminifera". NCBI Taxonomy Browser. 29178.