P2RX7
P2X purinoceptor 7 is a protein that in humans is encoded by the P2RX7 gene.[5][6]
| Part of a series on |
| Purinergic signalling |
|---|
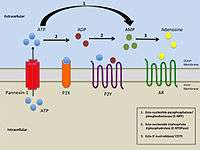 Simplified illustration of extracellular purinergic signalling |
| Concepts |
| Membrane transporters |
The product of this gene belongs to the family of purinoceptors for ATP. Multiple alternatively spliced variants which would encode different isoforms have been identified although some fit nonsense-mediated decay criteria.[7]
The receptor is found in the central and peripheral nervous systems, in microglia, in macrophages, in uterine endometrium, and in the retina.[8][9][10][11][12][13][14] The P2X7 receptor also serves as a pattern recognition receptor for extracellular ATP-mediated apoptotic cell death,[15][16][17] regulation of receptor trafficking,[18] mast cell degranulation,[19][20] and inflammation.[21][19][20][22]
Structure and kinetics
The P2X7 subunits can form homomeric receptors only with a typical P2X receptor structure.[23] The P2X7 receptor is a ligand-gated cation channel that opens in response to ATP binding and leads to cell depolarization. The P2X7 receptor requires higher levels of ATP than other P2X receptors; however, the response can be potentiated by reducing the concentration of divalent cations such as calcium or magnesium.[8][24] Continued binding leads to increased permeability to N-methyl-D-glucamine (NMDG+).[24] P2X7 receptors do not become desensitized readily and continued signaling leads to the aforementioned increased permeability and an increase in current amplitude.[24]
Pharmacology
Agonists
P2X7 receptors respond to BzATP more readily than ATP.[24] ADP and AMP are weak agonists of P2X7 receptors, but a brief exposure to ATP can increase their effectiveness.[24] Glutathione has been proposed to act as a P2X7 receptor agonist when present at milimolar levels, inducing calcium transients and GABA release from retinal cells.[10][9]
Antagonists
The P2X7 receptor current can be blocked by zinc, calcium, magnesium, and copper.[24] P2X7 receptors are sensitive to pyridoxalphosphate-6-azophenyl-2',4'-disulphonic acid (PPADS) and relatively insensitive to suramin, but the suramin analog, NF279, is much more effective. Oxidized ATP (OxATP) and Brilliant Blue G has also been used for blocking P2X7 in inflammation.[25][26] Other blockers include the large organic cations calmidazolium (a calmodulin antagonist) and KN-62 (a CaM kinase II antagonist).[24]
Receptor trafficking
In microglia, P2X7 receptors are found mostly on the cell surface.[27] Conserved cysteine residues located in the carboxyl terminus seem to be important for receptor trafficking to the cell membrane.[28] These receptors are upregulated in response to peripheral nerve injury.[29]
In melanocytic cells P2X7 gene expression may be regulated by MITF.[30]
Recruitment of pannexin
Activation of the P2X7 receptor by ATP leads to recruitment of pannexin pores[31] which allow small molecules such as ATP to leak out of cells. This allows further activation of purinergic receptors and physiological responses such a spreading cytoplasmic waves of calcium.[32] Moreover, this could be responsible for ATP-dependent lysis of macrophages through the formation of membrane pores permeable to larger molecules.
Clinical significance
Neuropathic pain
Microglial P2X7 receptors are thought to be involved in neuropathic pain because blockade or deletion of P2X7 receptors results in decreased responses to pain, as demonstrated in vivo.[33][34] Moreover, P2X7 receptor signaling increases the release of proinflammatory molecules such as IL-1β, IL-6, and TNF-α.[35][36][37] In addition, P2X7 receptors have been linked to increases in proinflammatory cytokines such as CXCL2 and CCL3.[38][39] P2X7 receptors are also linked to P2X4 receptors, which are also associated with neuropathic pain mediated by microglia.[27]
Osteoporosis
Mutations in this gene have been associated to low lumbar spine bone mineral density and accelerated bone loss in post-menopausal women.[40]
Researches
Possible link to hepatic fibrosis
One study in mice showed that blockade of P2X7 receptors attenuates onset of liver fibrosis.[43]
See also
- Purinergic receptor
- P2X receptor
References
- GRCh38: Ensembl release 89: ENSG00000089041 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000029468 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Rassendren F, Buell GN, Virginio C, Collo G, North RA, Surprenant A (February 1997). "The permeabilizing ATP receptor, P2X7. Cloning and expression of a human cDNA". The Journal of Biological Chemistry. 272 (9): 5482–6. doi:10.1074/jbc.272.9.5482. PMID 9038151.
- Buell GN, Talabot F, Gos A, Lorenz J, Lai E, Morris MA, Antonarakis SE (Feb 1999). "Gene structure and chromosomal localization of the human P2X7 receptor". Receptors & Channels. 5 (6): 347–54. PMID 9826911.
- "Entrez Gene: P2RX7 purinergic receptor P2X, ligand-gated ion channel, 7".
- Faria RX, Freitas HR, Reis RA (June 2017). "P2X7 receptor large pore signaling in avian Müller glial cells". Journal of Bioenergetics and Biomembranes. 49 (3): 215–229. doi:10.1007/s10863-017-9717-9. PMID 28573491. S2CID 4122579.
- Freitas HR, Reis RA (February 2017). "7R activation on Müller glia". Neurogenesis. 4 (1): e1283188. doi:10.1080/23262133.2017.1283188. PMC 5305167. PMID 28229088.
- Freitas HR, Ferraz G, Ferreira GC, Ribeiro-Resende VT, Chiarini LB, do Nascimento JL, et al. (April 2016). "Glutathione-Induced Calcium Shifts in Chick Retinal Glial Cells". PLOS ONE. 11 (4): e0153677. Bibcode:2016PLoSO..1153677F. doi:10.1371/journal.pone.0153677. PMC 4831842. PMID 27078878.
- Deuchars SA, Atkinson L, Brooke RE, Musa H, Milligan CJ, Batten TF, et al. (September 2001). "Neuronal P2X7 receptors are targeted to presynaptic terminals in the central and peripheral nervous systems". The Journal of Neuroscience. 21 (18): 7143–52. doi:10.1523/JNEUROSCI.21-18-07143.2001. PMC 6762981. PMID 11549725.
- Collo G, Neidhart S, Kawashima E, Kosco-Vilbois M, North RA, Buell G (September 1997). "Tissue distribution of the P2X7 receptor". Neuropharmacology. 36 (9): 1277–83. doi:10.1016/S0028-3908(97)00140-8. PMID 9364482. S2CID 21491471.
- Slater NM, Barden JA, Murphy CR (June 2000). "Distributional changes of purinergic receptor subtypes (P2X 1-7) in uterine epithelial cells during early pregnancy". The Histochemical Journal. 32 (6): 365–72. doi:10.1023/A:1004017714702. PMID 10943851. S2CID 40282870.
- Ishii K, Kaneda M, Li H, Rockland KS, Hashikawa T (May 2003). "Neuron-specific distribution of P2X7 purinergic receptors in the monkey retina". The Journal of Comparative Neurology. 459 (3): 267–77. doi:10.1002/cne.10608. PMID 12655509.
- Freitas (2019). "Interaction between cannabinoid and nucleotide systems as a new mechanism of signaling in retinal cell death". Neural Regeneration Research. 14 (12): 2093–2094. doi:10.4103/1673-5374.262585. PMC 6788250. PMID 31397346.
- Freitas HR, Isaac AR, Silva TM, Diniz GO, Dos Santos Dabdab Y, Bockmann EC, et al. (September 2019). "Cannabinoids Induce Cell Death and Promote P2X7 Receptor Signaling in Retinal Glial Progenitors in Culture". Molecular Neurobiology. 56 (9): 6472–6486. doi:10.1007/s12035-019-1537-y. PMID 30838518. S2CID 71143662.
- Kawano A, Tsukimoto M, Noguchi T, Hotta N, Harada H, Takenouchi T, et al. (March 2012). "Involvement of P2X4 receptor in P2X7 receptor-dependent cell death of mouse macrophages". Biochemical and Biophysical Research Communications. 419 (2): 374–80. doi:10.1016/j.bbrc.2012.01.156. PMID 22349510.
- Qu Y, Dubyak GR (June 2009). "P2X7 receptors regulate multiple types of membrane trafficking responses and non-classical secretion pathways". Purinergic Signalling. 5 (2): 163–73. doi:10.1007/s11302-009-9132-8. PMC 2686822. PMID 19189228.
- Kurashima Y, Kiyono H (March 2014). "New era for mucosal mast cells: their roles in inflammation, allergic immune responses and adjuvant development". Experimental & Molecular Medicine. 46 (3): e83. doi:10.1038/emm.2014.7. PMC 3972796. PMID 24626169.
- Wareham KJ, Seward EP (June 2016). "P2X7 receptors induce degranulation in human mast cells". Purinergic Signalling. 12 (2): 235–46. doi:10.1007/s11302-016-9497-4. PMC 4854833. PMID 26910735.
- Gonzaga DT, Ferreira LB, Moreira Maramaldo Costa TE, von Ranke NL, Anastácio Furtado Pacheco P, Sposito Simões AP, et al. (October 2017). "1-Aryl-1H- and 2-aryl-2H-1,2,3-triazole derivatives blockade P2X7 receptor in vitro and inflammatory response in vivo". European Journal of Medicinal Chemistry. 139: 698–717. doi:10.1016/j.ejmech.2017.08.034. PMID 28858765.
- Russo MV, McGavern DB (October 2015). "Immune Surveillance of the CNS following Infection and Injury". Trends in Immunology. 36 (10): 637–650. doi:10.1016/j.it.2015.08.002. PMC 4592776. PMID 26431941.
- Torres GE, Egan TM, Voigt MM (March 1999). "Hetero-oligomeric assembly of P2X receptor subunits. Specificities exist with regard to possible partners". The Journal of Biological Chemistry. 274 (10): 6653–9. doi:10.1074/jbc.274.10.6653. PMID 10037762.
- North RA (October 2002). "Molecular physiology of P2X receptors". Physiological Reviews. 82 (4): 1013–67. doi:10.1152/physrev.00015.2002. PMID 12270951.
- Wang X, Arcuino G, Takano T, Lin J, Peng WG, Wan P, et al. (August 2004). "P2X7 receptor inhibition improves recovery after spinal cord injury". Nature Medicine. 10 (8): 821–7. doi:10.1038/nm1082. PMID 15258577. S2CID 23685403.
- Peng W, Cotrina ML, Han X, Yu H, Bekar L, Blum L, et al. (July 2009). "Systemic administration of an antagonist of the ATP-sensitive receptor P2X7 improves recovery after spinal cord injury". Proceedings of the National Academy of Sciences of the United States of America. 106 (30): 12489–93. doi:10.1073/pnas.0902531106. PMC 2718350. PMID 19666625.
- Boumechache M, Masin M, Edwardson JM, Górecki DC, Murrell-Lagnado R (May 2009). "Analysis of assembly and trafficking of native P2X4 and P2X7 receptor complexes in rodent immune cells". The Journal of Biological Chemistry. 284 (20): 13446–54. doi:10.1074/jbc.M901255200. PMC 2679444. PMID 19304656.
- Jindrichova M, Kuzyk P, Li S, Stojilkovic SS, Zemkova H (June 2012). "Conserved ectodomain cysteines are essential for rat P2X7 receptor trafficking". Purinergic Signalling. 8 (2): 317–25. doi:10.1007/s11302-012-9291-x. PMC 3350585. PMID 22286664.
- Kobayashi K, Takahashi E, Miyagawa Y, Yamanaka H, Noguchi K (October 2011). "Induction of the P2X7 receptor in spinal microglia in a neuropathic pain model". Neuroscience Letters. 504 (1): 57–61. doi:10.1016/j.neulet.2011.08.058. PMID 21924325. S2CID 32284927.
- Hoek KS, Schlegel NC, Eichhoff OM, Widmer DS, Praetorius C, Einarsson SO, et al. (December 2008). "Novel MITF targets identified using a two-step DNA microarray strategy". Pigment Cell & Melanoma Research. 21 (6): 665–76. doi:10.1111/j.1755-148X.2008.00505.x. PMID 19067971.
- Iglesias R, Locovei S, Roque A, Alberto AP, Dahl G, Spray DC, Scemes E (September 2008). "P2X7 receptor-Pannexin1 complex: pharmacology and signaling". American Journal of Physiology. Cell Physiology. 295 (3): C752-60. doi:10.1152/ajpcell.00228.2008. PMC 2544446. PMID 18596211.
- Boison D, Chen JF, Fredholm BB (July 2010). "Adenosine signaling and function in glial cells". Cell Death and Differentiation. 17 (7): 1071–82. doi:10.1038/cdd.2009.131. PMC 2885470. PMID 19763139.
- {{cite journal | vauthors = Honore P, Donnelly-Roberts D, Namovic MT, Hsieh G, Zhu CZ, Mikusa JP, Hernandez G, Zhong C, Gauvin DM, Chandran P, Harris R, Medrano AP, Carroll W, Marsh K, Sullivan JP, Faltynek CR, Jarvis MF | s2cid = 11352013 | display-authors = 6 | title = A-740003 [N-(1-{[(cyanoimino)(5-quinolinylamino) methyl]amino}-2,2-dimethylpropyl)-2-(3,4-dimethoxyphenyl)acetamide], a novel and selective P2X7 receptor antagonist, dose-dependently reduces neuropathic pain in the rat | journal = The Journal of Pharmacology and Experimental Therapeutics | volume = 319 | issue = 3 | pages = 1376–85 | date = December 2006 | pmid = 16982702 | doi = 10.1124/jpet.106.111559 }}
- Chessell IP, Hatcher JP, Bountra C, Michel AD, Hughes JP, Green P, et al. (April 2005). "Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain". Pain. 114 (3): 386–96. doi:10.1016/j.pain.2005.01.002. PMID 15777864. S2CID 21486673.
- Clark AK, Staniland AA, Marchand F, Kaan TK, McMahon SB, Malcangio M (January 2010). "P2X7-dependent release of interleukin-1beta and nociception in the spinal cord following lipopolysaccharide". The Journal of Neuroscience. 30 (2): 573–82. doi:10.1523/JNEUROSCI.3295-09.2010. PMC 2880485. PMID 20071520.
- Shigemoto-Mogami Y, Koizumi S, Tsuda M, Ohsawa K, Kohsaka S, Inoue K (September 2001). "Mechanisms underlying extracellular ATP-evoked interleukin-6 release in mouse microglial cell line, MG-5". Journal of Neurochemistry. 78 (6): 1339–49. doi:10.1046/j.1471-4159.2001.00514.x. PMID 11579142.
- Hide I, Tanaka M, Inoue A, Nakajima K, Kohsaka S, Inoue K, Nakata Y (September 2000). "Extracellular ATP triggers tumor necrosis factor-alpha release from rat microglia". Journal of Neurochemistry. 75 (3): 965–72. doi:10.1046/j.1471-4159.2000.0750965.x. PMID 10936177.
- Shiratori M, Tozaki-Saitoh H, Yoshitake M, Tsuda M, Inoue K (August 2010). "P2X7 receptor activation induces CXCL2 production in microglia through NFAT and PKC/MAPK pathways". Journal of Neurochemistry. 114 (3): 810–9. doi:10.1111/j.1471-4159.2010.06809.x. PMID 20477948.
- Kataoka A, Tozaki-Saitoh H, Koga Y, Tsuda M, Inoue K (January 2009). "Activation of P2X7 receptors induces CCL3 production in microglial cells through transcription factor NFAT". Journal of Neurochemistry. 108 (1): 115–25. doi:10.1111/j.1471-4159.2008.05744.x. PMID 19014371.
- Gartland A, Skarratt KK, Hocking LJ, Parsons C, Stokes L, Jørgensen NR, et al. (May 2012). "Polymorphisms in the P2X7 receptor gene are associated with low lumbar spine bone mineral density and accelerated bone loss in post-menopausal women". European Journal of Human Genetics. 20 (5): 559–64. doi:10.1038/ejhg.2011.245. PMC 3330223. PMID 22234152.
- "Silencing immune attacks in type 1 diabetes". June 10, 2013. Retrieved June 15, 2013.
- "Boston Children's Hospital Finds Root Cause of Diabetes". June 13, 2013. Retrieved June 15, 2013.
- Huang C, Yu W, Cui H, Wang Y, Zhang L, Han F, Huang T (January 2014). "P2X7 blockade attenuates mouse liver fibrosis". Molecular Medicine Reports. 9 (1): 57–62. doi:10.3892/mmr.2013.1807. PMID 24247209.
Further reading
- Gartland A, Buckley KA, Hipskind RA, Bowler WB, Gallagher JA (2003). "P2 receptors in bone--modulation of osteoclast formation and activity via P2X7 activation". Critical Reviews in Eukaryotic Gene Expression. 13 (2–4): 237–42. doi:10.1615/CritRevEukaryotGeneExpr.v13.i24.150. PMID 14696970.
- Gartland A, Buckley KA, Bowler WB, Gallagher JA (October 2003). "Blockade of the pore-forming P2X7 receptor inhibits formation of multinucleated human osteoclasts in vitro". Calcified Tissue International. 73 (4): 361–9. doi:10.1007/s00223-002-2098-y. PMID 12874700. S2CID 23793221.
- Bowler WB, Buckley KA, Gartland A, Hipskind RA, Bilbe G, Gallagher JA (May 2001). "Extracellular nucleotide signaling: a mechanism for integrating local and systemic responses in the activation of bone remodeling". Bone. 28 (5): 507–12. doi:10.1016/S8756-3282(01)00430-6. PMID 11344050.
- Gartland A, Hipskind RA, Gallagher JA, Bowler WB (May 2001). "Expression of a P2X7 receptor by a subpopulation of human osteoblasts". Journal of Bone and Mineral Research. 16 (5): 846–56. doi:10.1359/jbmr.2001.16.5.846. PMID 11341329.
- Gartland A, Buckley KA, Hipskind RA, Perry MJ, Tobias JH, Buell G, et al. (2003). "Multinucleated osteoclast formation in vivo and in vitro by P2X7 receptor-deficient mice". Critical Reviews in Eukaryotic Gene Expression. 13 (2–4): 243–53. doi:10.1615/CritRevEukaryotGeneExpr.v13.i24.160. PMID 14696971.
- Maruyama K, Sugano S (January 1994). "Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides". Gene. 138 (1–2): 171–4. doi:10.1016/0378-1119(94)90802-8. PMID 8125298.
- Suzuki Y, Yoshitomo-Nakagawa K, Maruyama K, Suyama A, Sugano S (October 1997). "Construction and characterization of a full length-enriched and a 5'-end-enriched cDNA library". Gene. 200 (1–2): 149–56. doi:10.1016/S0378-1119(97)00411-3. PMID 9373149.
- Gu BJ, Zhang W, Worthington RA, Sluyter R, Dao-Ung P, Petrou S, et al. (April 2001). "A Glu-496 to Ala polymorphism leads to loss of function of the human P2X7 receptor". The Journal of Biological Chemistry. 276 (14): 11135–42. doi:10.1074/jbc.M010353200. PMID 11150303.
- Kim M, Jiang LH, Wilson HL, North RA, Surprenant A (November 2001). "Proteomic and functional evidence for a P2X7 receptor signalling complex". The EMBO Journal. 20 (22): 6347–58. doi:10.1093/emboj/20.22.6347. PMC 125721. PMID 11707406.
- Worthington RA, Smart ML, Gu BJ, Williams DA, Petrou S, Wiley JS, Barden JA (February 2002). "Point mutations confer loss of ATP-induced human P2X(7) receptor function". FEBS Letters. 512 (1–3): 43–6. doi:10.1016/S0014-5793(01)03311-7. PMID 11852049.
- Wiley JS, Dao-Ung LP, Gu BJ, Sluyter R, Shemon AN, Li C, et al. (March 2002). "A loss-of-function polymorphic mutation in the cytolytic P2X7 receptor gene and chronic lymphocytic leukaemia: a molecular study". Lancet. 359 (9312): 1114–9. doi:10.1016/S0140-6736(02)08156-4. PMID 11943260. S2CID 6019286.
- Wilson HL, Wilson SA, Surprenant A, North RA (September 2002). "Epithelial membrane proteins induce membrane blebbing and interact with the P2X7 receptor C terminus". The Journal of Biological Chemistry. 277 (37): 34017–23. doi:10.1074/jbc.M205120200. PMID 12107182.
- Atkinson L, Milligan CJ, Buckley NJ, Deuchars J (November 2002). "An ATP-gated ion channel at the cell nucleus". Nature. 420 (6911): 42. doi:10.1038/420042a. PMID 12422208. S2CID 4313025.
- Sluyter R, Wiley JS (December 2002). "Extracellular adenosine 5'-triphosphate induces a loss of CD23 from human dendritic cells via activation of P2X7 receptors". International Immunology. 14 (12): 1415–21. doi:10.1093/intimm/dxf111. PMID 12456589.
- Strausberg RL, Feingold EA, Grouse LH, Derge JG, Klausner RD, Collins FS, et al. (December 2002). "Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences". Proceedings of the National Academy of Sciences of the United States of America. 99 (26): 16899–903. Bibcode:2002PNAS...9916899M. doi:10.1073/pnas.242603899. PMC 139241. PMID 12477932.
- Wiley JS, Dao-Ung LP, Li C, Shemon AN, Gu BJ, Smart ML, et al. (May 2003). "An Ile-568 to Asn polymorphism prevents normal trafficking and function of the human P2X7 receptor". The Journal of Biological Chemistry. 278 (19): 17108–13. doi:10.1074/jbc.M212759200. PMID 12586825.
- Barden JA, Sluyter R, Gu BJ, Wiley JS (March 2003). "Specific detection of non-functional human P2X(7) receptors in HEK293 cells and B-lymphocytes". FEBS Letters. 538 (1–3): 159–62. doi:10.1016/S0014-5793(03)00172-8. PMID 12633871.
- Verhoef PA, Estacion M, Schilling W, Dubyak GR (June 2003). "P2X7 receptor-dependent blebbing and the activation of Rho-effector kinases, caspases, and IL-1 beta release". Journal of Immunology. 170 (11): 5728–38. doi:10.4049/jimmunol.170.11.5728. PMID 12759456.
- Greig AV, Linge C, Terenghi G, McGrouther DA, Burnstock G (June 2003). "Purinergic receptors are part of a functional signaling system for proliferation and differentiation of human epidermal keratinocytes". The Journal of Investigative Dermatology. 120 (6): 1007–15. doi:10.1046/j.1523-1747.2003.12261.x. PMID 12787128.
- Denlinger LC, Sommer JA, Parker K, Gudipaty L, Fisette PL, Watters JW, et al. (August 2003). "Mutation of a dibasic amino acid motif within the C terminus of the P2X7 nucleotide receptor results in trafficking defects and impaired function". Journal of Immunology. 171 (3): 1304–11. doi:10.4049/jimmunol.171.3.1304. PMID 12874219.
External links
- P2RX7+protein,+human at the US National Library of Medicine Medical Subject Headings (MeSH)
This article incorporates text from the United States National Library of Medicine, which is in the public domain.