Estradiol dienantate
Estradiol dienanthate (EDE), sold under the brand names Climacteron among others, is a long-acting estrogen medication which was previously used in menopausal hormone therapy for women and to suppress lactation in women.[1][2][3][4] It was formulated in combination with estradiol benzoate (EB), a short-acting estrogen, and testosterone enanthate benzilic acid hydrazone (TEBH), a long-acting androgen/anabolic steroid.[2][3][4] EDE has not been made available for medical use alone.[5] The medication, in combination with EB and TEBH, was given by injection into muscle once or at regular intervals, for instance once every 6 weeks.[6][7][8]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Climacteron, Amenose, Lactimex, Lactostat (all combinations) |
| Other names | Estradiol dienantate; EDE; EDEn; E2-EDN; Estradiol diheptanoate; Estra-1,3,5(10)-triene-3,17β-diol 3,17β-diheptanoate |
| Routes of administration | Intramuscular injection |
| Drug class | Estrogen; Estrogen ester |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ECHA InfoCard | 100.028.903 |
| Chemical and physical data | |
| Formula | C32H48O4 |
| Molar mass | 496.732 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Side effects of EDE include breast tenderness, breast enlargement, nausea, headache, and fluid retention.[9] EDE is a synthetic estrogen and hence is an agonist of the estrogen receptor, the biological target of estrogens like estradiol.[10][11] It is an estrogen ester and a prodrug of estradiol in the body.[11][10] Because of this, it is considered to be a natural and bioidentical form of estrogen.[11]
EDE was first described by 1959.[12][13] It was previously available in Canada and Germany but was discontinued by 2005.[14][15][16] The medication is no longer available in any form.[5]
Medical uses
EDE, a long-acting estrogen, was used in combination with EB, a short-acting estrogen, and TEBH, a long-acting androgen/anabolic steroid, in menopausal hormone therapy in perimenopausal, postmenopausal, hypogonadal, and oophorectomized women, as well as for suppression of lactation in postpartum women.[6][8][7]
Available forms
EDE was available only in combination EB and TEBH.[17] The combination was available in two different dose forms, one for menopausal hormone therapy (brand names Climacteron, Amenose) and the other for lactation suppression (brand names Lactimex, Lactostat). Climacteron and Amenose contained 1.0 mg EB, 7.5 mg EDE, and 150 mg TEBH (69 mg free testosterone) and was given by repeated intramuscular injection at regular intervals.[6][18][8] Lactimex and Lactostat contained 6 mg EB, 15 mg EDE, and 300 mg TEBH in 2 mL of corn oil and was administered as a single intramuscular injection after childbirth or during breastfeeding.[7][19][20][21]
| Route | Ingredient | Form | Dose | Major brand names |
|---|---|---|---|---|
| Oral | Estradiol | Tablet | 0.1, 0.2, 0.5, 1, 2, or 4 mg per tablet | Estrace, Ovocyclin |
| Estradiol acetatea | Tablet | 0.45, 0.9, or 1.8 mg per tablet | Femtrace | |
| Estradiol valerate | Tablet | 0.5, 1, 2, or 4 mg per tablet | Progynova | |
| Sublingual | Estradiola | Tablet | 0.125, 0.25, or 1 mg per tablet | Diogynets, Estradiol Membrettes |
| Intranasal | Estradiola | Nasal spray | 150 µg per spray (60 sprays per bottle) | Aerodiol |
| Transdermal | Estradiol | Patch | 14, 25, 37.5, 50, 60, 75, or 100 µg E2 per day for 3–4 or 7 days | Climara, Estraderm, Vivelle |
| Gel dispenser | 0.06% (0.87 or 1.25 g gel or 0.52 or 0.75 mg E2 per activation) | Elestrin, EstroGel | ||
| Gel packet | 0.1% (0.25, 0.5, or 1 g gel or 0.25, 0.5, or 1.0 mg E2 per packet) | DiviGel, Sandrena | ||
| Emulsion | 0.25% (1.74 g emulsion or 4.35 mg E2 or 25 µg/day E2 per pouch) | Estrasorb | ||
| Spray | 1.53 mg per spray | Evamist | ||
| Vaginal | Estradiol | Tablet | 10 or 25 µg per tablet | Vagifem |
| Cream | 0.01% (0.1 mg E2 per 1 g cream) | Estrace | ||
| Suppositorya | 4 or 40 μg per suppository | Ovocyclin | ||
| Insert | 4 or 10 µg per insert (daily for 2 weeks then twice weekly) | Imvexxy | ||
| Ring | 2 mg per ring (7.5 µg/day E2 for 3 months) | Estring | ||
| Estradiol acetate | Ring | 12.4 or 24.8 mg per ring (50 or 100 µg/day E2 for 3 months) | Femring | |
| Estradiol benzoatea | Tablet | 0.125 mg | Malun 25 | |
| Injection (IM or SC) | Estradiol | Aqueous suspensiona | 0.22, 0.25, 0.44, 0.5, 1.0, 1.1, or 2.0 mg/mL | Aquadiol, Diogyn, Progynon |
| Microspheres | 1 mg/mL | Juvenum E | ||
| Estradiol benzoate | Oil solution | 0.167, 0.2, 0.333, 1, 1.67, 2, 5, 10, 20, or 25 mg/mL | Progynon-B | |
| Aqueous suspensiona | 5 mg/mL | Agofollin Depot, Ovocyclin M | ||
| Estradiol cypionate | Oil solution | 1, 3, or 5 mg/mL | Depo-Estradiol | |
| Aqueous suspension | 5 mg/0.5 mL (available only with a progestin) | Cyclofem, Lunelle | ||
| Estradiol dipropionatea | Oil solution | 0.1, 0.2, 0.5, 1, 2.5, or 5 mg/mL | Di-Ovocylin, Progynon-DP | |
| Estradiol enanthate | Oil solution | 5 or 10 mg/mL (available only with a progestin) | Perlutal, Topasel | |
| Estradiol undecylatea | Oil solution | 100 mg/mL | Delestrec, Progynon Depot 100 | |
| Estradiol valerate | Oil solution | 5, 10, 20, or 40 mg/mL | Delestrogen, Progynon Depot | |
| Polyestradiol phosphatea | Aqueous solution | 40 or 80 mg per vial/ampoule | Estradurin | |
| Implant | Estradiola | Pellet | 20, 25, 50, or 100 mg per pellet (usually every 6 months) | Estradiol Implants, Meno-Implant |
| Abbreviations: E2 = Estradiol. Footnotes: a = Discontinued or mostly discontinued. Notes: (1): This table mostly does not include combination products, for instance estradiol formulated in combination with a progestogen or androgen. (2): This table does not include compounded estradiol products; only approved pharmaceutical preparations are included. (3): The availability of pharmaceutical estradiol products differs by country (see Estradiol (medication) § Availability). (4): Some of these formulations and doses have been marketed previously but may no longer be available. Sources: See template. | ||||
Pharmacology
Pharmacodynamics
EDE is an estradiol ester, or a prodrug of estradiol.[11][10] As such, it is an estrogen, or an agonist of the estrogen receptors.[11][10] EDE is of about 82% higher molecular weight than estradiol due to the presence of its C3 and C17β heptanoate (enanthate) esters. Because EDE is a prodrug of estradiol, it is considered to be a natural and bioidentical form of estrogen.[11]
| Estrogen | Form | Major brand names | EPD | CIC-D | Duration | |
|---|---|---|---|---|---|---|
| Estradiol | Aqueous solution | – | ? | – | <1 day | |
| Oil solution | Estradiol | 40–60 mg | – | 1–2 mg ≈ 1–2 days | ||
| Aqueous suspension | Aquadiol, Diogyn, Progynon, Mego-E | ? | 3.5 mg | 0.5–2 mg ≈ 2–7 days; 3.5 mg ≈ >5 days | ||
| Microspheres | Juvenum-E, Juvenum | ? | – | 1 mg ≈ 30 days | ||
| Estradiol benzoate | Oil solution | Progynon-B | 25–35 mg | – | 1.66 mg ≈ 2–3 days; 5 mg ≈ 3–6 days | |
| Aqueous suspension | Agofollin-Depot, Ovocyclin M | 20 mg | – | 10 mg ≈ 16–21 days | ||
| Emulsion | Menformon-Emulsion, Di-Pro-Emulsion | ? | – | 10 mg ≈ 14–21 days | ||
| Estradiol dipropionate | Oil solution | Agofollin, Di-Ovocylin, Progynon DP | 25–30 mg | – | 5 mg ≈ 5–8 days | |
| Estradiol valerate | Oil solution | Delestrogen, Progynon Depot, Mesigyna | 20–30 mg | 5 mg | 5 mg ≈ 7–8 days; 10 mg ≈ 10–14 days; 40 mg ≈ 14–21 days; 100 mg ≈ 21–28 days | |
| Estradiol benzoate butyrate | Oil solution | Redimen, Soluna, Unijab | ? | 10 mg | 10 mg ≈ 21 days | |
| Estradiol cypionate | Oil solution | Depo-Estradiol, Depofemin | 20–30 mg | – | 5 mg ≈ 11–14 days | |
| Aqueous suspension | Cyclofem, Lunelle | ? | 5 mg | 5 mg ≈ 14–24 days | ||
| Estradiol enanthate | Oil solution | Perlutal, Topasel, Yectames | ? | 5–10 mg | 10 mg ≈ 20–30 days | |
| Estradiol dienanthate | Oil solution | Climacteron, Lactimex, Lactostat | ? | – | 7.5 mg ≈ >40 days | |
| Estradiol undecylate | Oil solution | Delestrec, Progynon Depot 100 | ? | – | 10–20 mg ≈ 40–60 days; 25–50 mg ≈ 60–120 days | |
| Polyestradiol phosphate | Aqueous solution | Estradurin | 40–60 mg | – | 40 mg ≈ 30 days; 80 mg ≈ 60 days; 160 mg ≈ 120 days | |
| Estrone | Oil solution | Estrone, Kestrin, Theelin | ? | – | 1–2 mg ≈ 2–3 days | |
| Aqueous suspension | Estrone Aq. Susp., Kestrone, Theelin Aq. | ? | – | 0.1–2 mg ≈ 2–7 days | ||
| Estriol | Oil solution | – | ? | – | 1–2 mg ≈ 1–4 days | |
| Polyestriol phosphate | Aqueous solution | Gynäsan, Klimadurin, Triodurin | ? | – | 50 mg ≈ 30 days; 80 mg ≈ 60 days | |
| Notes: All aqueous suspensions are of microcrystalline particle size. Estradiol production during the menstrual cycle is 30–640 µg/day (6.4–8.6 mg total per month or cycle). The vaginal epithelium maturation dosage of estradiol benzoate or estradiol valerate has been reported as 5 to 7 mg/week. An effective ovulation-inhibiting dose of estradiol undecylate is 20–30 mg/month. Sources: See template. | ||||||
Pharmacokinetics
Estradiol and testosterone levels following a single intramuscular injection of Climacteron (including 1 mg EB, 7.5 mg EDE, and 150 mg TEBH equivalent to 69 mg free testosterone) versus 10 mg estradiol valerate have been studied over 28 days.[22][23]
| Estrogen | Form | Major brand names | EPD | CIC-D | Duration | |
|---|---|---|---|---|---|---|
| Estradiol | Aqueous solution | – | ? | – | <1 day | |
| Oil solution | Estradiol | 40–60 mg | – | 1–2 mg ≈ 1–2 days | ||
| Aqueous suspension | Aquadiol, Diogyn, Progynon, Mego-E | ? | 3.5 mg | 0.5–2 mg ≈ 2–7 days; 3.5 mg ≈ >5 days | ||
| Microspheres | Juvenum-E, Juvenum | ? | – | 1 mg ≈ 30 days | ||
| Estradiol benzoate | Oil solution | Progynon-B | 25–35 mg | – | 1.66 mg ≈ 2–3 days; 5 mg ≈ 3–6 days | |
| Aqueous suspension | Agofollin-Depot, Ovocyclin M | 20 mg | – | 10 mg ≈ 16–21 days | ||
| Emulsion | Menformon-Emulsion, Di-Pro-Emulsion | ? | – | 10 mg ≈ 14–21 days | ||
| Estradiol dipropionate | Oil solution | Agofollin, Di-Ovocylin, Progynon DP | 25–30 mg | – | 5 mg ≈ 5–8 days | |
| Estradiol valerate | Oil solution | Delestrogen, Progynon Depot, Mesigyna | 20–30 mg | 5 mg | 5 mg ≈ 7–8 days; 10 mg ≈ 10–14 days; 40 mg ≈ 14–21 days; 100 mg ≈ 21–28 days | |
| Estradiol benzoate butyrate | Oil solution | Redimen, Soluna, Unijab | ? | 10 mg | 10 mg ≈ 21 days | |
| Estradiol cypionate | Oil solution | Depo-Estradiol, Depofemin | 20–30 mg | – | 5 mg ≈ 11–14 days | |
| Aqueous suspension | Cyclofem, Lunelle | ? | 5 mg | 5 mg ≈ 14–24 days | ||
| Estradiol enanthate | Oil solution | Perlutal, Topasel, Yectames | ? | 5–10 mg | 10 mg ≈ 20–30 days | |
| Estradiol dienanthate | Oil solution | Climacteron, Lactimex, Lactostat | ? | – | 7.5 mg ≈ >40 days | |
| Estradiol undecylate | Oil solution | Delestrec, Progynon Depot 100 | ? | – | 10–20 mg ≈ 40–60 days; 25–50 mg ≈ 60–120 days | |
| Polyestradiol phosphate | Aqueous solution | Estradurin | 40–60 mg | – | 40 mg ≈ 30 days; 80 mg ≈ 60 days; 160 mg ≈ 120 days | |
| Estrone | Oil solution | Estrone, Kestrin, Theelin | ? | – | 1–2 mg ≈ 2–3 days | |
| Aqueous suspension | Estrone Aq. Susp., Kestrone, Theelin Aq. | ? | – | 0.1–2 mg ≈ 2–7 days | ||
| Estriol | Oil solution | – | ? | – | 1–2 mg ≈ 1–4 days | |
| Polyestriol phosphate | Aqueous solution | Gynäsan, Klimadurin, Triodurin | ? | – | 50 mg ≈ 30 days; 80 mg ≈ 60 days | |
| Notes: All aqueous suspensions are of microcrystalline particle size. Estradiol production during the menstrual cycle is 30–640 µg/day (6.4–8.6 mg total per month or cycle). The vaginal epithelium maturation dosage of estradiol benzoate or estradiol valerate has been reported as 5 to 7 mg/week. An effective ovulation-inhibiting dose of estradiol undecylate is 20–30 mg/month. Sources: See template. | ||||||
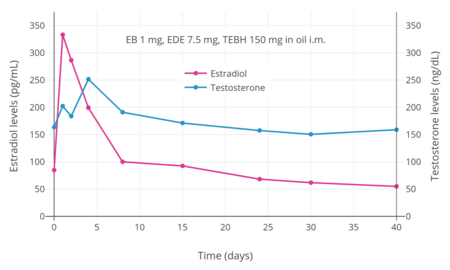 Estradiol and testosterone levels after an intramuscular injection of 1 mg estradiol benzoate, 7.5 mg estradiol dienanthate, and 150 mg testosterone enanthate benzilic acid hydrazone in oil (brand name Climacteron) in ovariectomized women.[24] Assays were performed using immunoassays.[24] Source was Sherwin (1987).[24]
Estradiol and testosterone levels after an intramuscular injection of 1 mg estradiol benzoate, 7.5 mg estradiol dienanthate, and 150 mg testosterone enanthate benzilic acid hydrazone in oil (brand name Climacteron) in ovariectomized women.[24] Assays were performed using immunoassays.[24] Source was Sherwin (1987).[24]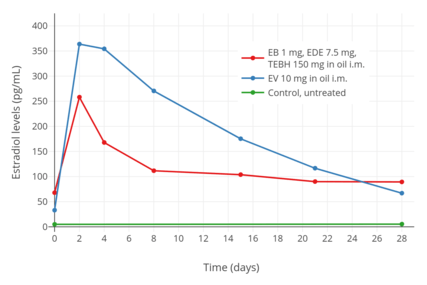 Estradiol levels after an intramuscular injection of 10 mg estradiol valerate in oil or Climacteron (1 mg estradiol benzoate, 7.5 mg estradiol dienanthate in oil) in ovariectomized women.[22] Assays were performed using RIA.[22] Source was Sherwin et al. (1987).[22]
Estradiol levels after an intramuscular injection of 10 mg estradiol valerate in oil or Climacteron (1 mg estradiol benzoate, 7.5 mg estradiol dienanthate in oil) in ovariectomized women.[22] Assays were performed using RIA.[22] Source was Sherwin et al. (1987).[22]
Chemistry
EDE is a synthetic estrane steroid and the C3 and C17β heptanoate (enanthate) diester of estradiol.[1] It is also known as estradiol 3,17β-heptanoate or as estra-1,3,5(10)-triene-3,17β-diol 3,17β-diheptanoate.[1] EDE is structurally related to estradiol enanthate (estradiol 17β-heptanoate), which has a single heptanoate ester rather than two.[1]
| Estrogen | Structure | Ester(s) | Relative mol. weight | Relative E2 contentb | logPc | ||||
|---|---|---|---|---|---|---|---|---|---|
| Position(s) | Moiet(ies) | Type | Lengtha | ||||||
| Estradiol | – | – | – | – | 1.00 | 1.00 | 4.0 | ||
| Estradiol acetate | C3 | Ethanoic acid | Straight-chain fatty acid | 2 | 1.15 | 0.87 | 4.2 | ||
| Estradiol benzoate | C3 | Benzenecarboxylic acid | Aromatic fatty acid | – (~4–5) | 1.38 | 0.72 | 4.7 | ||
| Estradiol dipropionate | C3, C17β | Propanoic acid (×2) | Straight-chain fatty acid | 3 (×2) | 1.41 | 0.71 | 4.9 | ||
| Estradiol valerate | C17β | Pentanoic acid | Straight-chain fatty acid | 5 | 1.31 | 0.76 | 5.6–6.3 | ||
| Estradiol benzoate butyrate | C3, C17β | Benzoic acid, butyric acid | Mixed fatty acid | – (~6, 2) | 1.64 | 0.61 | 6.3 | ||
| Estradiol cypionate | C17β | Cyclopentylpropanoic acid | Aromatic fatty acid | – (~6) | 1.46 | 0.69 | 6.9 | ||
| Estradiol enanthate | C17β | Heptanoic acid | Straight-chain fatty acid | 7 | 1.41 | 0.71 | 6.7–7.3 | ||
| Estradiol dienanthate | C3, C17β | Heptanoic acid (×2) | Straight-chain fatty acid | 7 (×2) | 1.82 | 0.55 | 8.1–10.4 | ||
| Estradiol undecylate | C17β | Undecanoic acid | Straight-chain fatty acid | 11 | 1.62 | 0.62 | 9.2–9.8 | ||
| Estradiol stearate | C17β | Octadecanoic acid | Straight-chain fatty acid | 18 | 1.98 | 0.51 | 12.2–12.4 | ||
| Estradiol distearate | C3, C17β | Octadecanoic acid (×2) | Straight-chain fatty acid | 18 (×2) | 2.96 | 0.34 | 20.2 | ||
| Estradiol sulfate | C3 | Sulfuric acid | Water-soluble conjugate | – | 1.29 | 0.77 | 0.3–3.8 | ||
| Estradiol glucuronide | C17β | Glucuronic acid | Water-soluble conjugate | – | 1.65 | 0.61 | 2.1–2.7 | ||
| Estramustine phosphated | C3, C17β | Normustine, phosphoric acid | Water-soluble conjugate | – | 1.91 | 0.52 | 2.9–5.0 | ||
| Polyestradiol phosphatee | C3–C17β | Phosphoric acid | Water-soluble conjugate | – | 1.23f | 0.81f | 2.9g | ||
| Footnotes: a = Length of ester in carbon atoms for straight-chain fatty acids or approximate length of ester in carbon atoms for aromatic fatty acids. b = Relative estradiol content by weight (i.e., relative estrogenic exposure). c = Experimental or predicted octanol/water partition coefficient (i.e., lipophilicity/hydrophobicity). Retrieved from PubChem, ChemSpider, and DrugBank. d = Also known as estradiol normustine phosphate. e = Polymer of estradiol phosphate (~13 repeat units). f = Relative molecular weight or estradiol content per repeat unit. g = logP of repeat unit (i.e., estradiol phosphate). Sources: See individual articles. | |||||||||
Society and culture
Brand names
EDE was marketed in combination with EB and TEBH under the brand names Climacteron, Amenose, Lactimex, and Lactostat.[17][8]
Availability
EDE is no longer available but was previously used in Canada, Germany and other countries.[5][17][7][21]
References
- J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. p. 898. ISBN 978-1-4757-2085-3.
- Ginsburg, Elizabeth S. (1999). "Androgen Replacement in Postmenopausal Women". In Seifer, David B.; Kennard, Elizabeth A. (eds.). Menopause. 18. pp. 209–219. doi:10.1007/978-1-59259-246-3_13. ISBN 978-1-61737-129-5.
- Robert B. Greenblatt; William E. Barfield; Edwin C. Jungck (January 1962). "The treatment of the menopause". Can Med Assoc J. 86 (3): 113–4. PMC 1848811. PMID 13901504.
- David B. Seifer (27 July 1999). Menopause: Endocrinology and Management. Springer Science & Business Media. pp. 183–. doi:10.1007/978-1-59259-246-3. ISBN 978-1-59259-246-3.
- https://www.drugs.com/international/estradiol.html
- "Climacteron Drug Information, Professional". Drugs.com. Retrieved 2 June 2019.
- Geburtshilfe und Frauenheilkunde: Ergebnisse der Forschung für die Praxis. Georg Thieme Verlag. 1969. p. 387,390.
[Kelly and Primose and Dodek found the following androgen-estrogen combination to be particularly effective and well-tolerated: 300 mg 3-benzilic acid hydrazone-testosterone-17-enanthate, 15 mg estradiol di-enanthate, 6 mg estradiol benzoate in 2 ml corn oil. This product is sold in Germany under the name Lactimex and has been clinically examined by us.] [...] Of 1200 postpartum patients one quarter stopped breast feeding for a variety of reasons and received an injection of Lactimex (Protina: Benzil acid hydrazon-testosteron-oenanthat 300 mg, Oestradiol-di-oenanthat 15 mg and Oestradiol-benzoate 6 mg in 1.0 ml of oil). In 76% of cases one injection was sufficient and the remaining 24% required a second injection. A second injection was required rarer if the first injection had been longer after delivery. A higher dosage of Lactimex was not necessary in cases with a preceding medical induction with intraveinous Oxytocin (Orasthin). Mothers who had been treated postpartum with methylergobasin did not as often require a second injection. No localized or generalized adverse reaction to the drug was noticed.
- Bundesverband der Pharmazeutischen Industrie (Germany) (1974). Rote Liste: Verzeichnis pharmazeutischer Spezialpräparate. Editio Cantor.
49035 В Amenose® Rp Ampullen Zus.: 1 Amp. 1 ml enth.: Benzilsäurehydrazid-N-testosteron-hydrazon-17-oenanthat 150 mg, Oestradiol-di-oenanthat 7.5 mg. Oestradiolbenzoat 1 mg in öl-Lösg. Ind.: Androgen-Oestrogen-Gemisch. Gegen Ausfallserscheinungen im Klimakterium und nach Ovarektomie. Osteoporose. Kontraind.: A 90, О 5 Dos.: Durchschnittl. alle 6 Wochen 1 Amp. im. 1 Amp. I ml 6.75 3 Amp 17.40 AP.: 10 Amp.
- Amit K. Ghosh (23 September 2010). Mayo Clinic Internal Medicine Board Review. OUP USA. pp. 222–. ISBN 978-0-19-975569-1.
- Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 Suppl 1: 3–63. doi:10.1080/13697130500148875. PMID 16112947.
- Michael Oettel; Ekkehard Schillinger (6 December 2012). Estrogens and Antiestrogens II: Pharmacology and Clinical Application of Estrogens and Antiestrogen. Springer Science & Business Media. p. 261. ISBN 978-3-642-60107-1.
Natural estrogens considered here include: [...] Esters of 17β-estradiol, such as estradiol valerate, estradiol benzoate and estradiol cypionate. Esterification aims at either better absorption after oral administration or a sustained release from the depot after intramuscular administration. During absorption, the esters are cleaved by endogenous esterases and the pharmacologically active 17β-estradiol is released; therefore, the esters are considered as natural estrogens.
- Vademecum International. J. Morgan Jones Publications. 1959. pp. 121–122.
- Kelly MJ, Primrose T (December 1960). "Evaluation of a new preparation for the suppression of lactation". Can Med Assoc J. 83: 1240–2. PMC 1938994. PMID 13752392.
- Al-Imari L, Wolfman WL (September 2012). "The safety of testosterone therapy in women". J Obstet Gynaecol Can. 34 (9): 859–865. doi:10.1016/S1701-2163(16)35385-3. PMID 22971455.
- Lexchin, Joel (2010). "Drug safety and Health Canada". International Journal of Risk & Safety in Medicine. 22 (1): 41–53. doi:10.3233/JRS-2010-0490.
- "Wayback Machine" (PDF). web.archive.org. 11 January 2013. Retrieved 2 June 2019.
- http://www.micromedexsolutions.com/micromedex2/
- Sherwin BB (1988). "Estrogen and/or androgen replacement therapy and cognitive functioning in surgically menopausal women". Psychoneuroendocrinology. 13 (4): 345–57. doi:10.1016/0306-4530(88)90060-1. PMID 3067252.
- Zentralblatt für Gynäkologie. J. A. Barth. 1971.
The preparation Lactimex (300 mg 3-benzyl hydrazone-testosterone-17-enanthate + 15 mg estradiol-dienanthate + 6 mg estradiol benzoate in 2 ml corn oil) was injected. [...]
- Helmuth Vorherr (2 December 2012). The Breast: Morphology, Physiology, and Lactation. Elsevier Science. pp. 201–. ISBN 978-0-323-15726-1.
- Compendium of Pharmaceuticals and Specialties. Canadian Pharmaceutical Association. 1983. ISBN 978-0-919115-04-0.
LACTOSTAT [...] Each 2 mL of injectable solution contains testosteorne enanthate benzilic acid hydrazone 300 mg, estradiol dienanthate 15 mg, estradiol benzoate 6 mg, benzyl alcohol 7.5% as preservative, benzyl benzoate 0.75 mg, corn oil q.s. Available in 2 mL ampuls, boxes of 25.
- Sherwin BB, Gelfand MM, Schucher R, Gabor J (February 1987). "Postmenopausal estrogen and androgen replacement and lipoprotein lipid concentrations". Am. J. Obstet. Gynecol. 156 (2): 414–9. doi:10.1016/0002-9378(87)90295-X. PMID 3826177.
- Sherwin BB (1988). "Affective changes with estrogen and androgen replacement therapy in surgically menopausal women". J Affect Disord. 14 (2): 177–87. doi:10.1016/0165-0327(88)90061-4. PMID 2966832.
- Sherwin, Barbara B.; Gelfand, Morrie M. (1987). "Individual differences in mood with menopausal replacement therapy: possible role of sex hormone-binding globulin". Journal of Psychosomatic Obstetrics & Gynecology. 6 (2): 121–131. doi:10.3109/01674828709016773. ISSN 0167-482X.