Epstein–Barr virus
The Epstein–Barr virus (EBV), formally called Human gammaherpesvirus 4, is one of the nine known human herpesvirus types in the herpes family, and is one of the most common viruses in humans.
| Human gammaherpesvirus 4 | |
|---|---|
| Electron micrograph of two Epstein–Barr virions (viral particles) showing round capsids loosely surrounded by the membrane envelope | |
| Virus classification | |
| (unranked): | Virus |
| Realm: | Duplodnaviria |
| Kingdom: | Heunggongvirae |
| Phylum: | Peploviricota |
| Class: | Herviviricetes |
| Order: | Herpesvirales |
| Family: | Herpesviridae |
| Genus: | Lymphocryptovirus |
| Species: | Human gammaherpesvirus 4 |
| Synonyms[1] | |
| |
It is best known as the cause of infectious mononucleosis ("mono" or "glandular fever"). It is also associated with various non-malignant, premalignant, and malignant Epstein–Barr virus-associated lymphoproliferative diseases such as Burkitt lymphoma, hemophagocytic lymphohistiocytosis,[2] and Hodgkin's lymphoma; non-lymphoid malignancies such as gastric cancer and nasopharyngeal carcinoma; and conditions associated with human immunodeficiency virus such as hairy leukoplakia and central nervous system lymphomas.[3][4] The virus is also associated with the childhood disorders of Alice in Wonderland syndrome[5] and acute cerebellar ataxia[6] and, based on some evidence, higher risks of developing certain autoimmune diseases,[7] especially dermatomyositis, systemic lupus erythematosus, rheumatoid arthritis, Sjögren's syndrome,[8][9] and multiple sclerosis.[10][11][12] About 200,000 cancer cases per year are thought to be attributable to EBV.[13][14]
Infection with EBV occurs by the oral transfer of saliva[15] and genital secretions.
Most people become infected with EBV and gain adaptive immunity. In the United States, about half of all five-year-old children and about 90% of adults have evidence of previous infection.[16] Infants become susceptible to EBV as soon as maternal antibody protection disappears. Many children become infected with EBV, and these infections usually cause no symptoms or are indistinguishable from the other mild, brief illnesses of childhood. In the United States and other developed countries, many people are not infected with EBV in their childhood years.[17] When infection with EBV occurs during adolescence, it causes infectious mononucleosis 35 to 50% of the time.[18]
EBV infects B cells of the immune system and epithelial cells. Once EBV's initial lytic infection is brought under control, EBV latency persists in the individual's B cells for the rest of their life.[15][19]
Signs and symptoms
Children who contract EBV exhibit few symptoms or may even appear asymptomatic, but when EBV is contracted as an adolescent or adult, it may cause fatigue, fever, inflamed throat, swollen lymph nodes in the neck, enlarged spleen, swollen liver, or rash.[20]. Post-infectious Chronic Fatigue Syndrome has also been associated with Epstein-Barr infection.[21]
Virology
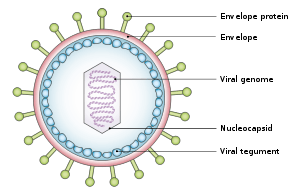
Structure and genome
The virus is about 122–180 nm in diameter and is composed of a double helix of deoxyribonucleic acid (DNA) which contains about 172,000 base pairs and 85 genes.[15] The DNA is surrounded by a protein nucleocapsid, which is surrounded by a tegument made of protein, which in turn is surrounded by an envelope containing both lipids[22] and surface projections of glycoproteins, which are essential to infection of the host cell.[22] In July 2020, a team of researchers reported the first complete atomic model of the nucleocapsid of the virus. This "first complete atomic model [includes] the icosahedral capsid, the capsid-associated tegument complex (CATC) and the dodecameric portal--the viral genome translocation apparatus." [23]
Tropism
The term viral tropism refers to which cell types that EBV infects. EBV can infect different cell types, including B cells and epithelial cells.[24]
The viral three-part glycoprotein complexes of gHgL gp42 mediate B cell membrane fusion; although the two-part complexes of gHgL mediate epithelial cell membrane fusion. EBV that are made in the B cells have low numbers of gHgLgp42 complexes, because these three-part complexes interact with Human-leukocyte-antigen class II molecules present in B cells in the endoplasmic reticulum and are degraded. In contrast, EBV from epithelial cells are rich in the three-part complexes because these cells do not normally contain HLA class II molecules. As a consequence, EBV made from B cells are more infectious to epithelial cells, and EBV made from epithelial cells are more infectious to B cells. Viruses lacking the gp42 portion are able to bind to human B cells, but unable to infect.[25]
Replication cycle
Entry to the cell
EBV can infect both B cells and epithelial cells. The mechanisms for entering these two cells are different.
To enter B cells, viral glycoprotein gp350 binds to cellular receptor CD21 (also known as CR2).[26] Then, viral glycoprotein gp42 interacts with cellular MHC class II molecules. This triggers fusion of the viral envelope with the cell membrane, allowing EBV to enter the B cell.[22] Human CD35, also known as complement receptor 1 (CR1), is an additional attachment factor for gp350/220, and can provide a route for entry of EBV into CD21-negative cells, including immature B-cells. EBV infection downregulates expression of CD35.[27]
To enter epithelial cells, viral protein BMRF-2 interacts with cellular β1 integrins. Then, viral protein gH/gL interacts with cellular αvβ6/αvβ8 integrins. This triggers fusion of the viral envelope with the epithelial cell membrane, allowing EBV to enter the epithelial cell.[22] Unlike B-cell entry, epithelial-cell entry is actually impeded by viral glycoprotein gp42.[26]
Once EBV enters the cell, the viral capsid dissolves and the viral genome is transported to the cell nucleus.
Lytic replication
The lytic cycle, or productive infection, results in the production of infectious virions. EBV can undergo lytic replication in both B cells and epithelial cells. In B cells, lytic replication normally only takes place after reactivation from latency. In epithelial cells, lytic replication often directly follows viral entry.[22]
For lytic replication to occur, the viral genome must be linear. The latent EBV genome is circular, so it must linearize in the process of lytic reactivation. During lytic replication, viral DNA polymerase is responsible for copying the viral genome. This contrasts with latency, in which host-cell DNA polymerase copies the viral genome.[22]
Lytic gene products are produced in three consecutive stages: immediate-early, early, and late.[22] Immediate-early lytic gene products act as transactivators, enhancing the expression of later lytic genes. Immediate-early lytic gene products include BZLF1 (also known as Zta, EB1, associated with its product gene ZEBRA) and BRLF1 (associated with its product gene Rta).[22] Early lytic gene products have many more functions, such as replication, metabolism, and blockade of antigen processing. Early lytic gene products include BNLF2.[22] Finally, late lytic gene products tend to be proteins with structural roles, such as VCA, which forms the viral capsid. Other late lytic gene products, such as BCRF1, help EBV evade the immune system.[22]
EGCG, a polyphenol in green tea, has shown in a study to inhibit EBV spontaneous lytic infection at the DNA, gene transcription, and protein levels in a time- and dose-dependent manner; the expression of EBV lytic genes Zta, Rta, and early antigen complex EA-D (induced by Rta), however, the highly stable EBNA-1 gene found across all stages of EBV infection is unaffected.[28] Specific inhibitors (to the pathways) suggest that Ras/MEK/MAPK pathway contributes to EBV lytic infection though BZLF1 and PI3-K pathway through BRLF1, the latter completely abrogating the ability of a BRLF1 adenovirus vector to induce the lytic form of EBV infection.[28] Additionally, the activation of some genes but not others is being studied to determine just how to induce immune destruction of latently infected B-cells by use of either TPA or sodium butyrate.[28]
Latency
Unlike lytic replication, latency does not result in production of virions.[22] Instead, the EBV genome circular DNA resides in the cell nucleus as an episome and is copied by cellular DNA polymerase.[22] In latency, only a portion of EBV's genes are expressed.[15][29] Latent EBV expresses its genes in one of three patterns, known as latency programs. EBV can latently persist within B cells and epithelial cells, but different latency programs are possible in the two types of cell.
EBV can exhibit one of three latency programs: Latency I, Latency II, or Latency III. Each latency program leads to the production of a limited, distinct set of viral proteins and viral RNAs.[30][31]
| Gene Expressed | EBNA-1 | EBNA-2 | EBNA-3A | EBNA-3B | EBNA-3C | EBNA-LP | LMP-1 | LMP-2A | LMP-2B | EBER |
|---|---|---|---|---|---|---|---|---|---|---|
| Product | Protein | Protein | Protein | Protein | Protein | Protein | Protein | Protein | Protein | ncRNAs |
| Latency I | + | – | – | – | – | – | – | – | – | + |
| Latency II | + | – | – | – | – | + | + | + | + | + |
| Latency III | + | + | + | + | + | + | + | + | + | + |
Also, a program is postulated in which all viral protein expression is shut off (Latency 0).
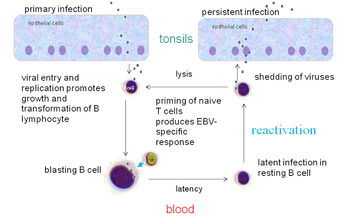
Within B cells, all three latency programs are possible.[15] EBV latency within B cells usually progresses from Latency III to Latency II to Latency I. Each stage of latency uniquely influences B cell behavior.[15] Upon infecting a resting naive B cell, EBV enters Latency III. The set of proteins and RNAs produced in Latency III transforms the B cell into a proliferating blast (also known as B cell activation).[15][22] Later, the virus restricts its gene expression and enters Latency II. The more limited set of proteins and RNAs produced in Latency II induces the B cell to differentiate into a memory B cell.[15][22] Finally, EBV restricts gene expression even further and enters Latency I. Expression of EBNA-1 allows the EBV genome to replicate when the memory B cell divides.[15][22]
Within epithelial cells, only Latency II is possible.
In primary infection, EBV replicates in oropharyngeal epithelial cells and establishes Latency III, II, and I infections in B-lymphocytes. EBV latent infection of B-lymphocytes is necessary for virus persistence, subsequent replication in epithelial cells, and release of infectious virus into saliva. EBV Latency III and II infections of B-lymphocytes, Latency II infection of oral epithelial cells, and Latency II infection of NK- or T-cell can result in malignancies, marked by uniform EBV genome presence and gene expression.[32]
Reactivation
Latent EBV in B cells can be reactivated to switch to lytic replication. This is known to happen in vivo, but what triggers it is not known precisely. In vitro, latent EBV in B cells can be reactivated by stimulating the B cell receptor, so reactivation in vivo probably takes place when latently infected B cells respond to unrelated infections.[22] In vitro, latent EBV in B cells can also be reactivated by treating the cells with sodium butyrate or 12-O-Tetradecanoylphorbol-13-acetate.
Transformation of B-lymphocytes
When EBV infects B cells in vitro, lymphoblastoid cell lines eventually emerge that are capable of indefinite growth. The growth transformation of these cell lines is the consequence of viral protein expression.
EBNA-2, EBNA-3C, and LMP-1 are essential for transformation, whereas EBNA-LP and the EBERs are not.[33]
Following natural infection with EBV, the virus is thought to execute some or all of its repertoire of gene expression programs to establish a persistent infection. Given the initial absence of host immunity, the lytic cycle produces large numbers of virions to infect other (presumably) B-lymphocytes within the host.
The latent programs reprogram and subvert infected B-lymphocytes to proliferate and bring infected cells to the sites at which the virus presumably persists. Eventually, when host immunity develops, the virus persists by turning off most (or possibly all) of its genes, only occasionally reactivating to produce fresh virions. A balance is eventually struck between occasional viral reactivation and host immune surveillance removing cells that activate viral gene expression.
The site of persistence of EBV may be bone marrow. EBV-positive patients who have had their own bone marrow replaced with bone marrow from an EBV-negative donor are found to be EBV-negative after transplantation.[34]
Latent antigens
All EBV nuclear proteins are produced by alternative splicing of a transcript starting at either the Cp or Wp promoters at the left end of the genome (in the conventional nomenclature). The genes are ordered EBNA-LP/EBNA-2/EBNA-3A/EBNA-3B/EBNA-3C/EBNA-1 within the genome.
The initiation codon of the EBNA-LP coding region is created by an alternate splice of the nuclear protein transcript. In the absence of this initiation codon, EBNA-2/EBNA-3A/EBNA-3B/EBNA-3C/EBNA-1 will be expressed depending on which of these genes is alternatively spliced into the transcript.
Protein/genes
| Protein/gene/antigen | Stage | Description |
|---|---|---|
| EBNA-1 | latent+lytic | EBNA-1 protein binds to a replication origin (oriP) within the viral genome and mediates replication and partitioning of the episome during division of the host cell. It is the only viral protein expressed during group I latency. |
| EBNA-2 | latent+lytic | EBNA-2 is the main viral transactivator. |
| EBNA-3 | latent+lytic | These genes also bind the host RBP-Jκ protein. |
| LMP-1 | latent | LMP-1 is a six-span transmembrane protein that is also essential for EBV-mediated growth transformation. |
| LMP-2 | latent | LMP-2A/LMP-2B are transmembrane proteins that act to block tyrosine kinase signaling. |
| EBER | latent | EBER-1/EBER-2 are small nuclear RNAs, which bind to certain nucleoprotein particles, enabling binding to PKR (dsRNA-dependent serin/threonin protein kinase), thus inhibiting its function. EBERs are by far the most abundant EBV products transcribed in EBV infected cells. They are commonly used as targets for the detection of EBV in histological tissues.[35] ER-particles also induce the production of IL-10, which enhances growth and inhibits cytotoxic T-cells. |
| v-snoRNA1 | latent | Epstein–Barr virus snoRNA1 is a box CD-snoRNA generated by the virus during latency. V-snoRNA1 may act as a miRNA-like precursor that is processed into 24 nucleotide sized RNA fragments that target the 3'UTR of viral DNA polymerase mRNA.[31] |
| ebv-sisRNA | latent | Ebv-sisRNA-1 is a stable intronic sequence RNA generated during latency program III. After the EBERs, it is the third-most abundant small RNA produced by the virus during this program.[36] |
| miRNAs | latent | EBV microRNAs are encoded by two transcripts, one set in the BART gene and one set near the BHRF1 cluster. The three BHRF1 pri-miRNAS (generating four miRNAs) are expressed during type III latency, whereas the large cluster of BART miRNAs (up to 20 miRNAs) are expressed during type II latency. The functions of these miRNAs are currently unknown. |
| EBV-EA | lytic | early antigen |
| EBV-MA | lytic | membrane antigen |
| EBV-VCA | lytic | viral capsid antigen |
| EBV-AN | lytic | alkaline nuclease[37] |
Subtypes of EBV
EBV can be divided into two major types, EBV type 1 and EBV type 2. These two subtypes have different EBNA-3 genes. As a result, the two subtypes differ in their transforming capabilities and reactivation ability. Type 1 is dominant throughout most of the world, but the two types are equally prevalent in Africa. One can distinguish EBV type 1 from EBV type 2 by cutting the viral genome with a restriction enzyme and comparing the resulting digestion patterns by gel electrophoresis.[22]
Role in disease
EBV has been implicated in several diseases, including infectious mononucleosis,[38] Burkitt's lymphoma,[39] Hodgkin's lymphoma,[40] stomach cancer,[13][41] nasopharyngeal carcinoma,[42] multiple sclerosis,[10][43][11] and lymphomatoid granulomatosis.[44] Specifically, EBV infected B-cells have been shown to reside within the brain lesions of multiple sclerosis patients.[11] Additional diseases that have been linked to EBV include Gianotti–Crosti syndrome, erythema multiforme, acute genital ulcers, oral hairy leukoplakia.[45] The viral infection is also associated with, and often contributes to the development of, a wide range of non-malignant lymphoproliferative diseases such as severe hypersensitivity Mosquito bite allergy reactions,[46] Epstein-Barr virus-positive mucocutaneous ulcers, and hydroa vacciniforme as well as malignant lymphoproliferative diseases such as Epstein–Barr virus-positive Burkitt lymphoma,[47] Epstein–Barr virus-positive Hodgkin lymphoma,[48] and primary effusion lymphoma.[49]
The Epstein–Barr virus has been implicated in disorders related to alpha-synuclein aggregation (e.g. Parkinson's disease, dementia with Lewy bodies, and multiple system atrophy).[50]
History
The Epstein–Barr virus was named after Michael Anthony Epstein (born 18 May 1921), now a professor emeritus at the University of Bristol, and Yvonne Barr (1932–2016), a 1966 Ph.D graduate from the University of London, who together discovered[51] and, in 1964, published on the existence of the virus.[52] In 1961, Epstein, a pathologist and expert electron microscopist, attended a lecture on "The Commonest Children's Cancer in Tropical Africa—A Hitherto Unrecognised Syndrome." This lecture, by Denis Parsons Burkitt, a surgeon practicing in Uganda, was the description of the "endemic variant" (pediatric form) of the disease that bears his name. In 1963, a specimen was sent from Uganda to Middlesex Hospital to be cultured. Virus particles were identified in the cultured cells, and the results were published in The Lancet in 1964 by Epstein, Bert Achong, and Barr. Cell lines were sent to Werner and Gertrude Henle at the Children's Hospital of Philadelphia who developed serological markers. In 1967, a technician in their laboratory developed mononucleosis and they were able to compare a stored serum sample, showing that antibodies to the virus developed.[53][54][55] In 1968, they discovered that EBV can directly immortalize B cells after infection, mimicking some forms of EBV-related infections,[56] and confirmed the link between the virus and infectious mononucleosis.[57]
Research
As a relatively complex virus, EBV is not yet fully understood. Laboratories around the world continue to study the virus and develop new ways to treat the diseases it causes. One popular way of studying EBV in vitro is to use bacterial artificial chromosomes.[58] Epstein–Barr virus can be maintained and manipulated in the laboratory in continual latency (a property shared with Kaposi's sarcoma-associated herpesvirus, another of the eight human herpesviruses). Although many viruses are assumed to have this property during infection of their natural hosts, there is not an easily managed system for studying this part of the viral lifecycle. Genomic studies of EBV have been able to explore lytic reactivation and regulation of the latent viral episome.[59]
Although under active research, an Epstein–Barr virus vaccine is not yet available. The development of an effective vaccine could prevent up to 200,000 cancers globally per year.[13] Like other human herpesvirus' Epstein-Barr might allow eradication via a course of the pro-drug Valaciclovir, but further research is needed to determine if eradication is actually achievable.[60]
See also
- Epstein–Barr virus infection
- Epstein–Barr virus-associated lymphoproliferative diseases
- James Corson Niederman, the physician who proved how the Epstein–Barr virus is transmitted in infectious mononucleosis
References
- "ICTV Taxonomy history: Human gammaherpesvirus 4". International Committee on Taxonomy of Viruses (ICTV). Retrieved 10 January 2019.
- Rezk SA, Zhao X, Weiss LM (September 2018). "Epstein-Barr virus (EBV)-associated lymphoid proliferations, a 2018 update". Human Pathology. 79: 18–41. doi:10.1016/j.humpath.2018.05.020. PMID 29885408.
- Maeda E, Akahane M, Kiryu S, Kato N, Yoshikawa T, Hayashi N, et al. (January 2009). "Spectrum of Epstein-Barr virus-related diseases: a pictorial review". Japanese Journal of Radiology. 27 (1): 4–19. doi:10.1007/s11604-008-0291-2. PMID 19373526.
- Cherry-Peppers G, Daniels CO, Meeks V, Sanders CF, Reznik D (February 2003). "Oral manifestations in the era of HAART". Journal of the National Medical Association. 95 (2 Suppl 2): 21S–32S. PMC 2568277. PMID 12656429.
- Mastria G, Mancini V, Viganò A, Di Piero V (2016). "Alice in Wonderland Syndrome: A Clinical and Pathophysiological Review". BioMed Research International. 2016: 8243145. doi:10.1155/2016/8243145. PMC 5223006. PMID 28116304.
- Nussinovitch M, Prais D, Volovitz B, Shapiro R, Amir J (September 2003). "Post-infectious acute cerebellar ataxia in children". Clinical Pediatrics. 42 (7): 581–4. doi:10.1177/000992280304200702. PMID 14552515.
- Toussirot E, Roudier J (October 2008). "Epstein-Barr virus in autoimmune diseases". Best Practice & Research. Clinical Rheumatology. 22 (5): 883–96. doi:10.1016/j.berh.2008.09.007. PMID 19028369.
- Dreyfus DH (December 2011). "Autoimmune disease: A role for new anti-viral therapies?". Autoimmunity Reviews. 11 (2): 88–97. doi:10.1016/j.autrev.2011.08.005. PMID 21871974.
- Pender MP (2012). "CD8+ T-Cell Deficiency, Epstein-Barr Virus Infection, Vitamin D Deficiency, and Steps to Autoimmunity: A Unifying Hypothesis". Autoimmune Diseases. 2012: 189096. doi:10.1155/2012/189096. PMC 3270541. PMID 22312480.
- Ascherio A, Munger KL (September 2010). "Epstein-barr virus infection and multiple sclerosis: a review". Journal of Neuroimmune Pharmacology. 5 (3): 271–7. doi:10.1007/s11481-010-9201-3. PMID 20369303.
- Moreno MA, Or-Geva N, Aftab BT, Khanna R, Croze E, Steinman L, Han MH (July 2018). "Molecular signature of Epstein-Barr virus infection in MS brain lesions". Neurology. 5 (4): e466. doi:10.1212/NXI.0000000000000466. PMC 5994704. PMID 29892607.
- Hassani A, Corboy JR, Al-Salam S, Khan G (2018). "Epstein-Barr virus is present in the brain of most cases of multiple sclerosis and may engage more than just B cells". PLOS ONE. 13 (2): e0192109. Bibcode:2018PLoSO..1392109H. doi:10.1371/journal.pone.0192109. PMC 5796799. PMID 29394264.
- "Developing a vaccine for the Epstein–Barr Virus could prevent up to 200,000 cancers globally say experts". 2014-03-24. Archived from the original on 2017-03-19.
- Khan G, Hashim MJ (2014). "Global burden of deaths from Epstein-Barr virus attributable malignancies 1990-2010". Infectious Agents and Cancer. 9 (1): 38. doi:10.1186/1750-9378-9-38. PMC 4253616. PMID 25473414.
- Amon W, Farrell PJ (November 2004). "Reactivation of Epstein-Barr virus from latency". Reviews in Medical Virology. 15 (3): 149–56. doi:10.1002/rmv.456. PMID 15546128.
- About 90% of adults have antibodies that show that they have a current or past EBV infection. Archived 2016-08-08 at the Wayback Machine National Center for Infectious Diseases
- ACP. "Epstein–Barr Virus Infections: Biology, Pathogenesis, and Management". ACP. Archived from the original on 2017-12-08. Retrieved 2017-12-08.
- CDC. "Epstein–Barr Virus and Infectious Mononucleosis". CDC. Archived from the original on 2012-04-20. Retrieved 2011-12-29.
- Khan G, Miyashita EM, Yang B, Babcock GJ, Thorley-Lawson DA (August 1996). "Is EBV persistence in vivo a model for B cell homeostasis?". Immunity. 5 (2): 173–9. doi:10.1016/s1074-7613(00)80493-8. PMID 8769480.
- "About Epstein–Barr Virus (EBV)". Centers for Disease Control and Prevention. 14 September 2016.
- Curr Clin Top Infect Dis. 1988;9:126-46.
- Odumade OA, Hogquist KA, Balfour HH (January 2011). "Progress and problems in understanding and managing primary Epstein-Barr virus infections". Clinical Microbiology Reviews. 24 (1): 193–209. doi:10.1128/CMR.00044-10. PMC 3021204. PMID 21233512.
- Scientists uncover first atomic structure of Epstein-Bar virus nucleocapsid
- Shannon-Lowe C, Rowe M (February 2014). "Epstein Barr virus entry; kissing and conjugation". Current Opinion in Virology. 4: 78–84. doi:10.1016/j.coviro.2013.12.001. PMID 24553068.
- Wang X, Hutt-Fletcher LM (January 1998). "Epstein-Barr virus lacking glycoprotein gp42 can bind to B cells but is not able to infect". Journal of Virology. 72 (1): 158–63. doi:10.1128/jvi.72.1.158-163.1998. PMC 109360. PMID 9420211.
- "Entrez Gene: CR2 complement component (3d/Epstein Barr virus) receptor 2". Archived from the original on 2010-12-05.
- Ogembo JG, Kannan L, Ghiran I, Nicholson-Weller A, Finberg RW, Tsokos GC, Fingeroth JD (February 2013). "Human complement receptor type 1/CD35 is an Epstein-Barr Virus receptor". Cell Reports. 3 (2): 371–85. doi:10.1016/j.celrep.2013.01.023. PMC 3633082. PMID 23416052.
- Liu S, Li H, Chen L, Yang L, Li L, Tao Y, et al. (March 2013). "(-)-Epigallocatechin-3-gallate inhibition of Epstein-Barr virus spontaneous lytic infection involves ERK1/2 and PI3-K/Akt signaling in EBV-positive cells". Carcinogenesis. 34 (3): 627–37. doi:10.1093/carcin/bgs364. PMID 23180656.
- Thorley-Lawson DA, Miyashita EM, Khan G (May 1996). "Epstein-Barr virus and the B cell: that's all it takes". Trends in Microbiology. 4 (5): 204–8. doi:10.1016/s0966-842x(96)90020-7. PMID 8727601.
- Calderwood MA, Venkatesan K, Xing L, Chase MR, Vazquez A, Holthaus AM, et al. (May 2007). "Epstein-Barr virus and virus human protein interaction maps". Proceedings of the National Academy of Sciences of the United States of America. 104 (18): 7606–11. Bibcode:2007PNAS..104.7606C. doi:10.1073/pnas.0702332104. PMC 1863443. PMID 17446270. The nomenclature used here is that of Kieff. Other laboratories use different nomenclatures.
- Hutzinger R, Feederle R, Mrazek J, Schiefermeier N, Balwierz PJ, Zavolan M, et al. (August 2009). "Expression and processing of a small nucleolar RNA from the Epstein-Barr virus genome". PLOS Pathogens. 5 (8): e1000547. doi:10.1371/journal.ppat.1000547. PMC 2718842. PMID 19680535.
- Robertson ES, ed. (2010). Epstein–Barr Virus: Latency and Transformation. Caister Academic Press. ISBN 978-1-904455-62-2.
- Yates JL, Warren N, Sugden B (1985). "Stable replication of plasmids derived from Epstein-Barr virus in various mammalian cells". Nature. 313 (6005): 812–5. Bibcode:1985Natur.313..812Y. doi:10.1038/313812a0. PMID 2983224.
- Gratama JW, Oosterveer MA, Zwaan FE, Lepoutre J, Klein G, Ernberg I (November 1988). "Eradication of Epstein-Barr virus by allogeneic bone marrow transplantation: implications for sites of viral latency". Proceedings of the National Academy of Sciences of the United States of America. 85 (22): 8693–6. Bibcode:1988PNAS...85.8693G. doi:10.1073/pnas.85.22.8693. PMC 282526. PMID 2847171.
- Khan G, Coates PJ, Kangro HO, Slavin G (July 1992). "Epstein Barr virus (EBV) encoded small RNAs: targets for detection by in situ hybridisation with oligonucleotide probes". Journal of Clinical Pathology. 45 (7): 616–20. doi:10.1136/jcp.45.7.616. PMC 495191. PMID 1325480.
- Moss WN, Steitz JA (August 2013). "Genome-wide analyses of Epstein-Barr virus reveal conserved RNA structures and a novel stable intronic sequence RNA". BMC Genomics. 14: 543. doi:10.1186/1471-2164-14-543. PMC 3751371. PMID 23937650.
- Buisson M, Géoui T, Flot D, Tarbouriech N, Ressing ME, Wiertz EJ, Burmeister WP (August 2009). "A bridge crosses the active-site canyon of the Epstein-Barr virus nuclease with DNase and RNase activities". Journal of Molecular Biology. 391 (4): 717–28. doi:10.1016/j.jmb.2009.06.034. PMID 19538972.
- Weiss LM, O'Malley D (January 2013). "Benign lymphadenopathies". Modern Pathology. 26 Suppl 1 (Supplement 1): S88-96. doi:10.1038/modpathol.2012.176. PMID 23281438.
- Pannone G, Zamparese R, Pace M, Pedicillo MC, Cagiano S, Somma P, et al. (2014). "The role of EBV in the pathogenesis of Burkitt's Lymphoma: an Italian hospital based survey". Infectious Agents and Cancer. 9 (1): 34. doi:10.1186/1750-9378-9-34. PMC 4216353. PMID 25364378.
- Gandhi MK, Tellam JT, Khanna R (May 2004). "Epstein-Barr virus-associated Hodgkin's lymphoma". British Journal of Haematology. 125 (3): 267–81. doi:10.1111/j.1365-2141.2004.04902.x. PMID 15086409.
- Yau, TO; Tang, CM; Yu, J (7 June 2014). "Epigenetic dysregulation in Epstein-Barr virus-associated gastric carcinoma: disease and treatments". World Journal of Gastroenterology. 20 (21): 6448–56. doi:10.3748/wjg.v20.i21.6448. PMC 4047330. PMID 24914366.
- Dogan S, Hedberg ML, Ferris RL, Rath TJ, Assaad AM, Chiosea SI (April 2014). "Human papillomavirus and Epstein-Barr virus in nasopharyngeal carcinoma in a low-incidence population". Head & Neck. 36 (4): 511–6. doi:10.1002/hed.23318. PMC 4656191. PMID 23780921.
- Mechelli R, Manzari C, Policano C, Annese A, Picardi E, Umeton R, et al. (March 2015). "Epstein-Barr virus genetic variants are associated with multiple sclerosis". Neurology. 84 (13): 1362–8. doi:10.1212/WNL.0000000000001420. PMC 4388746. PMID 25740864.
- Tagliavini E, Rossi G, Valli R, Zanelli M, Cadioli A, Mengoli MC, et al. (August 2013). "Lymphomatoid granulomatosis: a practical review for pathologists dealing with this rare pulmonary lymphoproliferative process". Pathologica. 105 (4): 111–6. PMID 24466760.
- Di Lernia V, Mansouri Y (October 2013). "Epstein-Barr virus and skin manifestations in childhood". International Journal of Dermatology. 52 (10): 1177–84. doi:10.1111/j.1365-4632.2012.05855.x. PMID 24073903.
- Kyriakidis I, Vasileiou E, Karastrati S, Tragiannidis A, Gompakis N, Hatzistilianou M (December 2016). "Primary EBV infection and hypersensitivity to mosquito bites: a case report". Virologica Sinica. 31 (6): 517–520. doi:10.1007/s12250-016-3868-4. PMID 27900557.
- Navari M, Etebari M, De Falco G, Ambrosio MR, Gibellini D, Leoncini L, Piccaluga PP (2015). "The presence of Epstein–Barr virus significantly impacts the transcriptional profile in immunodeficiency-associated Burkitt lymphoma". Frontiers in Microbiology. 6: 556. doi:10.3389/fmicb.2015.00556. PMC 4462103. PMID 26113842.
- Shannon-Lowe C, Rickinson AB, Bell AI (October 2017). "Epstein-Barr virus-associated lymphomas". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 372 (1732): 20160271. doi:10.1098/rstb.2016.0271. PMC 5597738. PMID 28893938.
- Arora N, Gupta A, Sadeghi N (July 2017). "Primary effusion lymphoma: current concepts and management". Current Opinion in Pulmonary Medicine. 23 (4): 365–370. doi:10.1097/MCP.0000000000000384. PMID 28399009.
- Woulfe J, Hoogendoorn H, Tarnopolsky M, Muñoz DG (November 2000). "Monoclonal antibodies against Epstein-Barr virus cross-react with alpha-synuclein in human brain". Neurology. 55 (9): 1398–401. doi:10.1212/WNL.55.9.1398. PMID 11087792.
- McGrath P (6 April 2014). "Cancer virus discovery helped by delayed flight". BBC News, Health. Archived from the original on 8 October 2015. Retrieved 4 November 2015.
- Epstein MA, Achong BG, Barr YM (March 1964). "Virus Particles in Cultured Lymphoblasts from Burkitt's Lymphoma". Lancet. 1 (7335): 702–3. doi:10.1016/s0140-6736(64)91524-7. PMID 14107961.
- Epstein MA (2005). "1. The origins of EBV research: discovery and characterization of the virus". In Robertson ES (ed.). Epstein–Barr Virus. Trowbridge: Cromwell Press. pp. 1–14. ISBN 978-1-904455-03-5. Retrieved September 18, 2010.
- Erle S. Robertson (2005). Epstein–Barr Virus. Horizon Scientific Press. p. 18. ISBN 978-1-904455-03-5. Retrieved 3 June 2012.
- Miller G (December 21, 2006). "Book Review: Epstein–Barr Virus". New England Journal of Medicine. 355 (25): 2708–2709. doi:10.1056/NEJMbkrev39523.
- Henle W, Henle G (1980). "Epidemiologic aspects of Epstein-Barr virus (EBV)-associated diseases". Annals of the New York Academy of Sciences. 354: 326–31. doi:10.1111/j.1749-6632.1980.tb27975.x. PMID 6261650.
- Young, LS (2009). Desk Encyclopedia of Human and Medical Virology. Boston: Academic Press. pp. 532–533.
- Delecluse HJ, Feederle R, Behrends U, Mautner J (December 2008). "Contribution of viral recombinants to the study of the immune response against the Epstein-Barr virus". Seminars in Cancer Biology. 18 (6): 409–15. doi:10.1016/j.semcancer.2008.09.001. PMID 18938248.
- Arvey A, Tempera I, Tsai K, Chen HS, Tikhmyanova N, Klichinsky M, et al. (August 2012). "An atlas of the Epstein-Barr virus transcriptome and epigenome reveals host-virus regulatory interactions". Cell Host & Microbe. 12 (2): 233–45. doi:10.1016/j.chom.2012.06.008. PMC 3424516. PMID 22901543.
- Hoshino Y, Katano H, Zou P, Hohman P, Marques A, Tyring SK, et al. (November 2009). "Long-term administration of valacyclovir reduces the number of Epstein-Barr virus (EBV)-infected B cells but not the number of EBV DNA copies per B cell in healthy volunteers". Journal of Virology. 83 (22): 11857–61. doi:10.1128/JVI.01005-09. PMC 2772668. PMID 19740997.
External links
External links


- Wikidata: topic (Scholia)