Duplodnaviria
Duplodnaviria is a realm of viruses that includes all double-stranded DNA viruses that encode the HK97 major capsid protein. The HK97 major capsid protein (HK97-MCP) is the primary component of the viral capsid, which stores the viral deoxyribonucleic acid (DNA). Viruses in the realm also share a number of other characteristics, such as an icosahedral capsid, an opening in the viral capsid called a portal, a protease enzyme that empties the inside of the capsid prior to DNA packaging, and a terminase enzyme that packages viral DNA into the capsid.
| Duplodnaviria | |
|---|---|
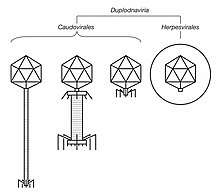 | |
| Illustrated sample of Duplodnaviria virions | |
| Virus classification | |
| (unranked): | Virus |
| Realm: | Duplodnaviria |
| Kingdom: | Heunggongvirae |
| Subtaxa | |
| Synonyms[1][2] | |
| |
Duplodnaviria was established in 2019 based on the shared characteristics of viruses in the realm. There are two groups of viruses in Duplodnaviria: tailed bacteriophages of the order Caudovirales, which infect prokaryotes, and herpesviruses of the order Herpesvirales, which infect animals. Tailed bacteriophages are very diverse and ubiquitous worldwide, and they may be the oldest lineage of viruses. Herpesviruses either share a common ancestor with tailed bacteriophages or are a breakaway group from within Caudovirales.
Tailed bacteriophages are important in marine ecology by recycling nutrients in organic material from their hosts and are the focus of much research, and herpesviruses are associated with a variety of diseases in animals, including humans. A common feature among viruses in Duplodnaviria is that many are able to persist in their host for long periods of time without replicating while still being able to resurface in the future. Example of this include the herpes simplex virus, which causes recurring infections, and the varicella zoster virus, which initially causes chickenpox early in life then shingles later in life.
Etymology
The name Duplodnaviria is a portmanteau of duplo, the Latin word for double, dna, from deoxyribonucleic acid (DNA), referencing that all members of the realm at founding had double-stranded DNA genomes, and -viria, which is the suffix used for virus realms. Duplodnaviria is monotypic, having only one kingdom, Heunggongvirae, so both the realm and kingdom have the same definition. Heunggongvirae takes the first part of its name from Cantonese 香港 [Hēunggóng], meaning and approximately pronounced "Hong Kong", which is a reference to Escherichia virus HK97, the founding member of the HK97 (Hong Kong 97) fold MCP viruses, and the suffix -virae, which is the suffix used for virus kingdoms.[2]
Characteristics
All viruses in Duplodnaviria contain a distinct icosahedral capsid that is composed of a major capsid protein that contains a unique folded structure, called the HK97 fold, named after the folded structure of the MCP of the bacteriophage species Escherichia virus HK97. Despite having significant variation across Duplodnaviria, the base structure of the protein is retained among all species in the realm. Other shared proteins that involve the structure and assembly of capsids include a portal protein that the opening of the capsid is made of, a protease that empties the capsid before DNA is inserted, and the terminase enzyme that inserts the DNA into the capsid.[1][2][3]
After HK97-MCP has been synthesized by the host cell's ribosomes, the viral capsid is assembled from it with the proteins bonding to each other. The inside of the capsid contains scaffold proteins that guide the geometric construction of the capsid. In the absence of separate scaffolding proteins, the delta domain of HK97-MCP, which faces toward the inside of the capsid, acts as a scaffold protein.[1][3][4]
A cylindrical opening in the capsid, called a portal, that serves as the entrance and exit for viral DNA is created with portal proteins at one of the 12 vertices of the capsid. The scaffold protein, which may be the delta domain of HK97-MCP, is removed from the inside of the capsid by the capsid maturation protease, which may also be a part of the scaffolding, breaking it and itself down to smaller molecules in a process called proteolysis that leaves the inside of the capsid empty.[3][4]
At the same time as capsid assembly, replication of the viral DNA occurs, creating concatemers, long molecules of DNA containing numerous copies of the viral genome. The enzyme terminase, made of two subunits, large and small, finds the viral DNA inside of the cell via the small subunit, cuts the concatemers, and creates the termini, or endings, of the genomes. Terminase recognizes a packaging signal in the genome and cuts the nucleic acid, creating a free end that it binds to.[3]
The terminase, now bound to the concatemer, attaches itself to the capsid portal and begins translocating the DNA from outside the capsid to the inside, using energy generated from ATP hydrolysis by the large subunit. As more DNA is inserted into the capsid, the capsid expands in size, becomes thinner, and its surface becomes flatter and more angular. Once the genome is completely inside, terminase cuts the concatemer again, completing packaging. Terminase then detaches itself from the portal and proceeds to repeat this process until all genomes in the concatemer have been packaged.[3]
For tailed bacteriophages, after DNA packaging, the tail of the virion, which was assembled separately, is attached to the capsid, commonly called the "head" of tailed bacteriophages, at the portal. Tailed bacteriophages also sometimes have "decoration" proteins that attach to the capsid's surface in order to reinforce the capsid's structure. After the virion is fully assembled inside the host cell, it leaves the cell.[3] Tailed bacteriophages leave the cell via lysis, rupturing of the cell membrane, that causes cell death,[5] and herpesviruses leave by budding from the host cell membrane, using the membrane as a viral envelope that covers the capsid.[6]
Phylogenetics
Tailed bacteriophages are potentially the oldest lineage of viruses in the world because they are ubiquitous worldwide, only infect prokaryotes, and have a high level of diversity. Their highly divergent virion structures may point to this or may indicate separate origins. The origin of Herpesvirales is unclear, but there are two likely scenarios. First, ancestral lineages of Caudovirales may have produced clades at various times that were capable of infecting eukaryotes, and the strong similarity that Herpesvirales has with Caudovirales may indicate that it is a more recent descendant of one such lineage. The second likely scenario is that Herpesvirales is a breakaway clade from within Caudovirales, which is supported by the Caudovirales family Myoviridae, and especially one of its subfamilies, Tevenvirinae, showing a relatively high genetic relation to herpesviruses based on certain protein amino acid sequences.[7] It has been suggested that Duplodnaviria predates the last universal common ancestor (LUCA) of cellular life and that viruses in the realm were present in the LUCA.[8]
Outside of Duplodnaviria, an HK97-like fold is only found in encapsulins, which are prokaryotic nanocompartments that encapsulate a variety of cargo proteins that are related to the oxidative stress response. High resolution images of encapsulins have also shown that they assemble into icosahedrons like the capsids of viruses in Duplodnaviria. However, the HK97-MCP in viruses is much more divergent and widespread than encapsulins, which form a narrow monophyletic clade. As such, it is more likely that encapsulins are derived from viruses than vice versa. An exception to this viewpoint is that encapsulins have also been identified in archaea of the phylum Crenarchaeota, which are not known to be infected by tailed bacteriophages, so the relation between encapsulins and Duplodnaviria remains unresolved.[9]
The ATPase subunit of Duplodnaviria terminases that generates energy for packaging viral DNA has the same general structural design of the P-loop fold as the packaging ATPases of double jelly roll viruses in the realm Varidnaviria but are otherwise not directly related to each other. While viruses in Duplodnaviria make use of the HK97 fold for their major capsid proteins, the major capsid proteins of viruses in Varidnaviria instead are marked by vertical single or double jelly roll folds.[2]
Classification
Duplodnaviria contains only one kingdom, and this kingdom is subdivided into two phyla that are monotypic down to the rank of order. This taxonomy can be visualized as follows:[10]
- Realm: Duplodnaviria
- Kindgom: Heunggongvirae
- Phylum: Peploviricota
- Class: Herviviricetes
- Order: Herpesvirales – the herpesviruses, which only infect animals (eukaryotes)
- Phylum: Uroviricota
- Class: Caudoviricetes
- Order: Caudovirales – the tailed bacteriophages, which only infect archaea and bacteria (prokaryotes)
As all viruses in the realm are double-stranded DNA (dsDNA) viruses, the realm belongs to Group I: dsDNA viruses of Baltimore classification, a classification system based on a virus's manner of messenger RNA (mRNA) production, often used alongside standard virus taxonomy, which is based on evolutionary history.[2] Realms are the highest level of taxonomy used for viruses and Duplodnaviria is one of four, the other three being Monodnaviria, Riboviria, and Varidnaviria.[10]
Interactions with hosts
Viral shunt
Tailed bacteriophages are ubiquitous worldwide and are a major cause of death among prokaryotes. Infection may lead to cell death via lysis, the rupturing of the cell membrane. As a result of lysis, organic material from the killed prokaryotes is released into the environment, contributing to a process called viral shunt. Tailed bacteriophages shunt nutrients from organic material away from higher trophic levels so that they can be consumed by organisms in lower trophic levels, which has the effects of recycling nutrients and promoting increased diversity among marine life.[11]
Disease
Herpesviruses are associated with a wide range of diseases in their hosts, including a respiratory tract illness in chickens,[12] a respiratory and reproductive illness in cattle,[13] and tumors in sea turtles.[14] In humans, herpesviruses usually cause various epithelial diseases such as herpes simplex, chickenpox and shingles, and Kaposi's sarcoma.[15][16][17] Initial infection causes acute symptoms and leads to lifelong infection via latency. Herpesviruses may emerge from their latency to cause illnesses, which may have severe symptoms such as encephalitis and pneumonia.[18][19]
Latency
Viruses in Duplodnaviria have two different types of replication cycles, called the lytic cycle, whereby infection leads directly to virion formation and exit from the host cell, and the lysogenic cycle, whereby a latent infection retains the viral DNA inside of the host cell without virion formation, either as an episome or via integration into the host cell's DNA, with the possibility of returning to the lytic cycle in the future. Viruses that can replicate through the lysogenic cycle are called temperate or lysogenic viruses. Tailed bacteriophages vary in their temperateness, whereas all herpesviruses are temperate and able to avoid detection by the host's immune system, causing lifelong infections.[20][21]
History
Tailed bacteriophages were discovered independently by Frederick Twort in 1915 and Félix d'Hérelle in 1917, and they have been the focus of much research since then.[22] Diseases in humans caused by herpesviruses have been recognized for much of recorded history, and person-to-person transmission of the herpes simplex virus, the first herpesvirus discovered, was first recognized in 1893 by Émile Vidal.[23][24]
Over time, the two groups were increasingly found to share many characteristics, and their genetic relation was formalized with the establishment of Duplodnaviria in 2019. The creation of the kingdom, phyla, and classes of the realm in the same year has also created a framework to more easily allow major reorganization of Caudovirales, which is growing in size significantly and which may require tailed bacteriophages to be promoted to the rank of class or higher.[2]
See also
References
- Suhanovsky MM, Teschke CM (May 2015). "Nature's favorite building block: Deciphering folding and capsid assembly of proteins with the HK97-fold". Virology. 479-480: 479–480. doi:10.1016/j.virol.2015.02.055. PMC 4424165. PMID 25864106.
- Koonin EV, Dolja VV, Krupovic M, Varsani A, Wolf YI, Yutin N, Zerbini M, Kuhn JH (18 October 2019). "Create a megataxonomic framework, filling all principal/primary taxonomic ranks, for dsDNA viruses encoding HK97-type major capsid proteins" (docx). International Committee on Taxonomy of Viruses. Retrieved 19 May 2020.
- Rao VB, Feiss M (November 2015). "Mechanisms of DNA Packaging by Large Double-Stranded DNA Viruses". Annu Rev Virol. 2 (1): 351–378. doi:10.1146/annurev-virology-100114-055212. PMC 4785836. PMID 26958920.
- Duda RL, Oh B, Hendrix RW (9 August 2013). "Functional domains of the HK97 capsid maturation protease and the mechanisms of protein encapsidation". J Mol Biol. 425 (15): 2765–2781. doi:10.1016/j.jmb.2013.05.002. PMC 3709472. PMID 23688818.
- "Myoviridae". ViralZone. Swiss Institute of Bioinformatics. Retrieved 19 May 2020.
- "Herpesviridae". ViralZone. Swiss Institute of Bioinformatics. Retrieved 19 May 2020.
- Andrade-Martínez JS, Moreno-Gallego JL, Reyes A (August 2019). "Defining a Core Genome for the Herpesvirales and Exploring their Evolutionary Relationship with the Caudovirales" (PDF). Sci Rep. 9 (1): 11342. Bibcode:2019NatSR...911342A. doi:10.1038/s41598-019-47742-z. PMC 6683198. PMID 31383901. Retrieved 19 May 2020.
- Krupovic, M; Dolja, VV; Koonin, EV (14 July 2020). "The LUCA and its complex virome". Nat Rev Microbiol. doi:10.1038/s41579-020-0408-x. PMID 32665595.
- Krupovic M, Koonin EV (21 March 2017). "Multiple origins of viral capsid proteins from cellular ancestors". Proc Natl Acad Sci U S A. 114 (12): E2401–E2410. doi:10.1073/pnas.1621061114. PMC 5373398. PMID 28265094.
- "Virus Taxonomy: 2019 Release". talk.ictvonline.org. International Committee on Taxonomy of Viruses. Retrieved 25 April 2020.
- Wilhelm SW, Suttle CA (October 1999). "Viruses and Nutrient Cycles in the Sea: Viruses play critical roles in the structure and function of aquatic food webs". BioScience. 49 (10): 781–788. doi:10.2307/1313569. JSTOR 1313569. Retrieved 15 June 2020.
- Fuchs W, Veits J, Helferich D, Granzow H, Teifke JP, Mettenleiter TC (2007). "Molecular biology of avian infectious laryngotracheitis virus". Vet Res. 38 (2): 261–279. doi:10.1051/vetres:200657. PMID 17296156.
- Graham DA (2013). "Bovine herpes virus-1 (BoHV-1) in cattle–a review with emphasis on reproductive impacts and the emergence of infection in Ireland and the United Kingdom". Ir Vet J. 66 (1): 15. doi:10.1186/2046-0481-66-15. PMC 3750245. PMID 23916092.
- Jones K, Ariel E, Burgess G, Read M (June 2016). "A Review of Fibropapillomatosis in Green Turtles (Chelonia Mydas)". Vet J. 212: 48–57. doi:10.1016/j.tvjl.2015.10.041. PMID 27256025. Retrieved 15 June 2020.
- Kukhanova MK, Korovina AN, Kochetkov SN (December 2014). "Human herpes simplex virus: life cycle and development of inhibitors". Biochemistry (Mosc). 79 (13): 1635–1652. doi:10.1134/S0006297914130124. PMID 25749169.
- Gershon AA, Breuer J, Cohen JI, Cohrs RJ, Gershon MD, Gilden D, Grose C, Hambleton S, Kennedy PG, Oxman MN, Seward JF, Yamanishi K (2 July 2015). "Varicella zoster virus infection". Nat Rev Dis Primers. 1: 15016. doi:10.1038/nrdp.2015.16. PMC 5381807. PMID 27188665. Retrieved 9 August 2020.
- O'Leary JJ, Kennedy MM, McGee JO (February 1997). "Kaposi's sarcoma associated herpes virus (KSHV/HHV 8): epidemiology, molecular biology and tissue distribution". Mol Pathol. 50 (1): 4–8. doi:10.1136/mp.50.1.4. PMC 379571. PMID 9208806.
- Bradshaw MJ, Venkatesan A (July 2016). "Herpes Simplex Virus-1 Encephalitis in Adults: Pathophysiology, Diagnosis, and Management". Neurotherapeutics. 13 (3): 493–508. doi:10.1007/s13311-016-0433-7. PMC 4965403. PMID 27106239. Retrieved 9 August 2020.
- Sezgen E, An P, Winkler CA (23 July 2019). "Host Genetics of Cytomegalovirus Pathogenesis". Front Genet. 10: 616. doi:10.3389/fgene.2019.00616. PMC 6664682. PMID 31396258.
- Weidner-Glunde M, Kruminis-Kaszkiel E, Savanagoudar M (February 2020). "Herpesviral Latency—Common Themes". Pathogens. 9 (2): 125. doi:10.3390/pathogens9020125. PMC 7167855. PMID 32075270.
- "Virus latency". ViralZone. Swiss Institute of Bioinformatics. Retrieved 15 June 2020.
- Keen EC (January 2015). "A century of phage research: Bacteriophages and the shaping of modern biology". BioEssays. 37 (1): 6–9. doi:10.1002/bies.201400152. PMC 4418462. PMID 25521633.
- Hunt, R. D. (1993). Herpesviruses of Primates: An Introduction. In: Jones T.C., Mohr U., Hunt R.D. (eds) Nonhuman Primates I. Springer, Berlin, Heidelberg. p. 74–78. doi:10.1007/978-3-642-84906-0_11. ISBN 978-3-642-84906-0.
- Wildy P (1973) Herpes: history and classification. In: Kaplan AS, ed. The herpes-viruses. New York: Academic Press: 1-25. Accessed 15 June 2020.
Further reading
- Ward, C. W. (1993). "Progress towards a higher taxonomy of viruses". Research in Virology. 144 (6): 419–53. doi:10.1016/S0923-2516(06)80059-2. PMC 7135741. PMID 8140287.