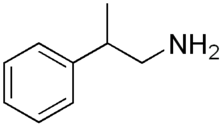
β-Methylphenethylamine
β-Methylphenethylamine (β-Me-PEA, BMPEA, or 1-amino-2-phenylpropane) is an organic compound of the phenethylamine class, and a positional isomer of the drug amphetamine, with which it shares some properties. In particular, both amphetamine and β-methylphenethylamine are human TAAR1 agonists.[1] In appearance, it is a colorless or yellowish liquid.
 | |
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| ECHA InfoCard | 100.008.619 |
| Chemical and physical data | |
| Formula | C9H13N |
| Molar mass | 135.210 g·mol−1 |
| 3D model (JSmol) | |
| Density | 0.93 g/cm3 |
| Boiling point | 80 °C (176 °F) (at 10 mm Hg) |
| |
| |
| (verify) | |
Relatively little information has been published about this substance. Hartung and Munch reported that it had good antihypotensive (pressor) activity in experimental animals, and that it was orally active. The MLD (minimum lethal dose) for the HCl salt was given as 500 mg/kg (rat, s.c.) and 50 mg/kg (rabbit, i.v.).[2]
A study by Graham and co-workers at the Upjohn Co., comparing many β-methylphenethylamines substituted on the benzene ring showed that β-methylphenethylamine itself had 1/700 x the pressor activity of epinephrine, corresponding to ~ 1/3 the potency of amphetamine. The β-methyl compound also had ~ 2 x the broncho-dilating power of amphetamine (as measured using the isolated rabbit lung), and an LD50 of 50 mg/kg (rat, i.v.).[3]
Synthesis
β-Methylphenethylamine can be made by the catalytic hydrogenation of 2-phenylpropionitrile with Pd/C in pure anhydrous ethanol containing three equivalents of HCl; the finished product is extracted as the HCl salt, m.p. 123-124°.[2]
Presence
In 2015, 52% of supplements labeled as containing Acacia rigidula were found to contain BMPEA.[4][5] Consumers following recommended maximum daily servings would consume a maximum of 94 mg of BMPEA per day.[4] In 2012, however, the FDA determined that BMPEA was not naturally present in Acacia rigidula leaves.[6] This question was litigated during the trial of Hi Tech Pharmaceuticals Inc vs. Cohen.[7][8] Despite US Food and Drug Administration warning letters, BMPEA remains present in dietary supplements.[9]
Safety
β-Methylphenethylamine was associated with a case of cerebral hemorrhage in a Swedish athlete and first time user. The female victim with no medical history had taken a Swedish food supplement with 290 mg β-methylphenethylamine per serving before commencing her usual exercises. After about 30 minutes the first symptoms appeared. The presence of the active ingredient was not declared on the label.[10] Use of β-Methylphenethylamine is also prohibited in sport.[11]
See also
References
- Wainscott DB, Little SP, Yin T, Tu Y, Rocco VP, He JX, Nelson DL (2007). "Pharmacologic characterization of the cloned human trace amine-associated receptor1 (TAAR1) and evidence for species differences with the rat TAAR1". The Journal of Pharmacology and Experimental Therapeutics. 320 (1): 475–85. doi:10.1124/jpet.106.112532. PMID 17038507. S2CID 10829497.
The effect of β-carbon substitution on the phenylethylamine side chain was also investigated (Table 3). A β-methyl substituent was well tolerated compared with β-PEA. In fact, S-(–)-β-methyl-β-PEA was as potent as β-PEA at human TAAR1.
- Hartung WH, Munch JC (1931). "Amino alcohols. VI. The preparation and pharmacodynamic activity of four isomeric phenylpropylamines". J. Am. Chem. Soc. 53 (5): 1875–9. doi:10.1021/ja01356a036.
- Graham BE, Cartland GF, Woodruff EH (1945). "Phenyl propyl and phenyl isopropyl amines. Changes in pharmacological action on substitution of phenyl nucleus and amino nitrogen". Ind. Eng. Chem. 37 (2): 149–51. doi:10.1021/ie50422a010.
- Cohen, Pieter A.; Bloszies, Clayton; Yee, Caleb; Gerona, Roy (2016). "An amphetamine isomer whose efficacy and safety in humans has never been studied, β-methylphenylethylamine (BMPEA), is found in multiple dietary supplements". Drug Testing and Analysis. 8 (3–4): 328–333. doi:10.1002/dta.1793. PMID 25847603.
- "Conflicts of Interest at the F.D.A." The New York Times. 13 April 2015. Retrieved 13 April 2015.
they identified BMPEA in 11 of 21 brands of supplements with acacia rigidula listed as an ingredient.
- Brenda Goodman (7 April 2015). "Untested Stimulant Still in Dietary Supplements". WebMD.
- Bagley, Nicolas; Carroll, Aaron E.; Cohen, Pieter A. (1 January 2018). "Scientific Trials—In the Laboratories, Not the Courts". JAMA Internal Medicine. 178 (1): 7–8. doi:10.1001/jamainternmed.2017.5730. PMID 29114742.
- "BMPEA and Acacia Rigidula: Hi-Tech Pharmaceuticals Fights Back". PricePlow. 26 October 2015. Retrieved 27 October 2015.
- Cohen, Pieter A.; Wen, Anita; Gerona, Roy (1 December 2018). "Prohibited Stimulants in Dietary Supplements After Enforcement Action by the US Food and Drug Administration". JAMA Internal Medicine. 178 (12): 1721–1723. doi:10.1001/jamainternmed.2018.4846. PMC 6583602. PMID 30422217.
- Cohen PA, Zeijlon R, Nardin R, Keizers PH, Venhuis BJ (2015). "Hemorrhagic Stroke Probably Caused by Exercise Combined With a Sports Supplement Containing β-Methylphenyl-ethylamine (BMPEA): A Case Report". Ann. Intern. Med. 162 (16): 879–80. doi:10.7326/L15-5101. PMID 26075771.
- Cholbiński P, Wicka M, Kowalczyk K, Jarek A, Kaliszewski P, Pokrywka A, Bulska E, Kwiatkowska D (2014). "Detection of β-methylphenethylamine, a novel doping substance, by means of UPLC/MS/MS". Anal Bioanal Chem. 406 (15): 3681–8. doi:10.1007/s00216-014-7728-5. PMC 4026626. PMID 24633566.