Thymidine kinase in clinical chemistry
Thymidine kinase is an enzyme, a phosphotransferase (a kinase): 2'-deoxythymidine kinase, ATP-thymidine 5'-phosphotransferase, EC 2.7.1.21 [1][2] that catalyzes the reaction:
Thd + ATP → TMP + ADP
where Thd is (deoxy)thymidine, ATP is adenosine 5’-triphosphate, TMP is (deoxy)thymidine 5’-phosphate and ADP is adenosine 5’-diphosphate. In clinical chemistry it has been suggested as a proliferation marker for prognosis, verification of diagnosis, control of treatment (particularly as a companion diagnostic) and follow-up of malignant disease. It is used mainly in relation to hematological malignancies but the developments of more sensitive assays have stimulated investigations for its use in relation to solid tumors.
History
The incorporation of thymidine in DNA was demonstrated around 1950.[3] Somewhat later, it was shown that this incorporation was preceded by phosphorylation[4] and around 1960, the enzyme responsible was purified and characterized. [5][6] The potential use as a tumor marker was suggested by Gronowitz et al.[7]
Biochemistry
Mammals have two isoenzymes that are chemically very different, Thymidine Kinase 1 (TK1) and Thymidine Kinase 2 (TK2). The former was first found in fetal tissue, the second was found to be more abundant in adult tissue, and so initially they were termed fetal and adult thymidine kinases. Soon it was shown that TK1 is present in the cytoplasm only in anticipation of cell division (cell cycle-dependent)[8][9] whereas the presence of TK2, which is located in the mitochondria, is cell cycle-independent.[10][11] TK1 is synthesized by the cell during the S phase of cell division. After cell division is completed, TK1 is degraded intracellularly, so that it does not pass into body fluids after normal cell division.[12] The TK enzyme suggested as a tumor marker is the cytosolic cell cycle dependent TK1. It is present during cell division in much higher concentrations than TK2 and it is released in quantities that completely dominate the thymidine kinase activity in blood and other body fluids.
In addition to cellular TKs, virus specific thymidine kinases have been identified in Herpes simplex virus, Varicella zoster virus and Epstein-Barr virus.[13][14][15][16][17][18][19] They differ biochemically from thymidine kinase from mammalian cells and are inhibited by specific inhibitors that do not influence the activity of mammalian thymidine kinases. Determination of viral thymidine kinase has been suggested for confirmation of diagnosis and for control of treatment of viral infections.
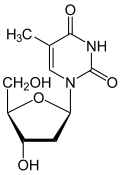
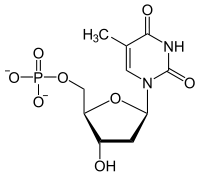
Thymidine reacts with ATP to give thymidine monophosphate and ADP.
Physiological context
Thymidine monophosphate, the product of the reaction catalyzed by thymidine kinase, is in turn phosphorylated to thymidine diphosphate by the enzyme thymidylate kinase and further to thymidine triphosphate by the enzyme nucleoside diphosphate kinase. The triphosphate is included in a DNA molecule, a reaction catalyzed by a DNA polymerase and a complementary DNA molecule (or an RNA molecule in the case of reverse transcriptase, an enzyme present in retrovirus). Thymidine monophosphate is produced by the cell in two different reactions - either by phosphorylation of thymidine as described above or by methylation of deoxyuridine monophosphate, a product of other metabolic pathways unrelated to thymidine, by the enzyme thymidylate synthase (De novo synthesis). This second route is used by the cell under normal conditions, and it is sufficient to supply thymidine monophosphate for DNA repair. When a cell prepares to divide, a complete new set-up of DNA is required, and the requirement for building blocks, including thymidine triphosphate, increases. Cells prepare for cell division by making some of the enzymes required during the division. They are not normally present in the cells and are down-regulated and degraded afterwards. Such enzymes are called salvage enzymes. Thymidine kinase 1 is such a salvage enzyme, whereas thymidine kinase 2 is not cell cycle-dependent.[20][21][22][23][24][25][26][27][28]
Thymidine kinase in serum
Background
Thymidine kinase is a salvage enzyme that is only present in anticipation of cell division. The enzyme is not set free from cells undergoing normal division where the cells have a special mechanism to degrade the proteins no longer needed after cell division is completed.[9] In normal subjects, the amount of thymidine kinase in serum or plasma is, therefore, very low. Tumor cells release enzyme to the circulation, probably in connection with the disruption of dead or dying tumor cells. The thymidine kinase level in serum, therefore, serves as a measure of malignant proliferation and, indirectly, as a measure of the aggressivity of the tumor. The form of enzyme present in the circulation does not correspond to the protein as encoded by the gene: the gene corresponds to a protein with molecular weight around 25 kD. It is a dimer with a molecular weight of around 50 kD, if activated by ATP a tetramer with molecular weight around 100 kD.[29] The main fraction of the active enzyme in the circulation has a molecular weight of 730 kD and is probably bound in a complex to other proteins.[30]
Measurement
Thymidine kinase 1 (TK1) levels in serum or plasma may be measured based on their enzymatic activity or in terms of mass using immunoassays. In enzyme activity assays, this is done by incubation of a serum sample with a substrate analogue. The oldest commercially available technique uses iodo-deoxyuridine (idoxuridine) wherein a methyl group in thymidine has been replaced with radioactive iodine.[31][32][33] This substrate is well accepted by the enzyme. The monophosphate of iodo deoxyuridine is adsorbed on aluminum oxide that is suspended in the incubation medium. After decantation and washing the radioactivity of the aluminum oxide gives a measure of the amount of thymidine kinase in the sample. Kits using this principle are commercially available from the companies Immunotech/Beckman and DiaSorin.
A non-radioactive assay method has been developed by the company Dia-Sorin. In this technique 3'-azido-2',3'-deoxythymidine (Zidovudine, AZT) is first phosphorylated to AZT 5'-monophosphate (AZTMP) by the TK1 in the sample. AZTMP is measured in an immunoassay with anti-AZTMP antibodies and AZTMP-labeled peroxidase. The assay runs in a closed system on the laboratory robot from DiaSorin.[34][35] The DiviTum assay from Biovica International uses another thymidine analogue, bromodeoxyuridine, as substrate to the enzyme. The product of the reaction is further phosphorylated to tri-phosphate and incorporated in DNA-strings of polythymidine. The polythymidine binds to strings of polyadenine coupled to the bottom of the wells in microtiter plates. There it is detected with an ELISA technique: The wells are filled with a solution of a monoclonal antibody to bromo-deoxyuridine. The monoclonal antibody has been bound (conjugated) to the enzyme alkaline phosphatase. After the unbound antibody-conjugate has been washed away, a solution of a substrate to the alkaline phosphatase, para-nitrophenylphosphate, is added. The product of the reaction, para-nitrophenol, is yellow at alkaline pH and can be measured by photometry.[36] This method has been evaluated against the previous radioactive technique. It is considerably more sensitive than the previous enzymatic methods and may be, therefore, more suitable for use with solid tumors where lower elevations of TK1 are found in body fluids. Comparisons of the methods have been published.[37][38] In the study by Nisman et al.,[37] while the Divitum was on the whole more sensitive than the Liaison method, the authors suggested that the Liaison method may have been more sensitive for the TK1 forms found in normal subjects. A continuous and homogeneous fluorescent method based on quenching technology has recently been published. This technique utilizes natural thymidine as substrate and can also determine deoxycytidine kinase simultaneously with TK1.[39]
Immunoassays enabling the direct determination of TK1 protein have now been developed.[40][41][42] Immunoassays have advantages over enzymme activity methods in that they can measure TK1 isoforms that are enzymatically inactive plus that they are unaffected by serum TK1 inhibitors.[41] The specific activity of serum TK1 differs between cancer types[43] and using an immunoassay method may aid in comparing TK1 levels between subjects and malignancy types. Due to the basic differences in assay methods, results obtained with TK1 activity assays and immunoassay may differ, e.g. an ELISA based on antibodies against the TK1 TK 210 epitope was shown to be twice as sensitive as a TK1 activity assay in distinguishing between healthy women and subjects with breast cancer[44]
Two immunoassays have been developed against the exposed ‘210’ epitope covering the C-terminal amino acid sequence 194-225,[45] a direct dot-blot assay with chemiluminescence end point[46] and a microtiter sandwich ELISA.[44] The dot-blot assay is a nitrocellulose membrane based assay with a chemiluminsecent substrate utilising a primary chicken IgY antibody and a secondary labeled anti-IgY antibody. In brief, the sample is spotted onto a nitrocellulose membrane where the proteins in the sample bind. After blocking, the membrane is incubated with a primary anti-TK1 antibody which binds to the TK1 on the membrane. After washing, a biotinylated second antibody directed against IgY antibodies is added followed by streptavidin labelled HRP and a chemiluminescent substrate. A microtiter ELISA based on monoclonal antibodies directed against the “210” epitope is available from AroCell. The AroCell TK 210 ELISA system uses a pre-treatment buffer to break up the high molecular weight TK1 complexes and expose the TK 210 epitope. The treated samples are added to a microtiter plate coated with anti TK 210 monoclonal antibodies. After incubating and washing, a second anti-TK 210 antibody labeled with biotin is added. After further washing, the color is developed with streptavidin labelled horse radish peroxidase with TMP as substrate.
A microchip electrophoresis immunoaffinity assay for determination of serum thymidine kinase concentration has been described. Its function was demonstrated using recombinant TK1. It is claimed to be fast and simple to perform.[47]
Serum thymidine kinase 1 in different malignancies
Hematologic malignancies
The most dramatic increases of serum TK1 are seen in hematologic malignancies. The increases seen in both TK1 activity and concentration are greater in hematologic malignancies compared with solid tumors.[43][48]
Non-Hodgkin lymphoma
The main use of serum TK1 activity assays is in non-Hodgkin lymphoma. This disease has a wide range of aggressivity, from slow-growing indolent disease that hardly requires treatment to highly aggressive, rapidly growing forms that should be treated urgently. This is reflected in the values of serum TK1 activity, that range from close to the normal range for slow-growing tumors to very high levels for rapidly growing forms.[7][49][50][51][52][53][54][55][56][57][58][59]
Leukemias
Leukemias normally do not normally present major diagnostic difficulties, as the microscopic analysis of the cells in blood usually provides unequivocal results. TK1 assays, however, may give supplementary information about the aggressivity and the risk for progression.[60][61][62][63][64][65][66][67]
Myeloma
Also myelomas often constitute a diagnostic challenge. The malignant cells are often not available for microscopic analysis, and the prognosis is often uncertain. Therefore, information on the prognosis may be essential in the decision of the treatment. Several studies verify the close connection between prognosis and thymidine kinase activity in myelomas.[38][68][69]
Myelodysplastic syndrome
A very interesting case is the myelodysplastic syndrome: Some rapidly change to acute leukemia, whereas others remain indolent for very long time. Identification of those tending to change to overt leukemia is important for the treatment. A relationship between the prognosis and the serum TK1 values has been demonstrated in myelodysplastic syndrome .[70][71]
Solid tumors
Increased serum TK1 levels may be found in subjects with solid tumors. The increases in serum TK1 activity levels in subjects with solid tumors are not as large as they are for hematologic malignancies. The first methods for determination of serum TK1 activity had a limited sensitivity. In the case of the methods employing radioactivity, one reason was that the quantity of radioactivity allowed by law in normal radioimmunoassay laboratories is strctly limited. The experimental method first developed by Gronowitz et al.[31] used quantities of radioisotope much higher than those used in commercial radioassays and, therefore, the sensitivity was sufficient to detect increases in serum TK1 in subjects with solid tumors. With commercial radioassays this was difficult, and the results were not very convincing. Later, more sensitive, non-radioactive techniques enabled the lower increases from solid tumors to be measured accurately. The lower TK1 concentrations and lower TK1 specific activity found in solid tumors may make TK1 immunoassays more suitable.[46][44]
Lung cancer
Lung cancer is one of the commonest mlignancies, both by incidence (about 15% for both men and women in USA and in Europe) and by mortality (25% for women and 30% for men). One major reason why the mortality is higher than the incidence is that lung cancer is mostly detected and diagnosed at a late stage. Early detection could reduce the mortality. Another reason is that lung cancer, particularly small cell lung cancer, is very aggressive with very low 5 year survival rates.
There are several reports of the utility of TK1 activity measurements in serum in lung cancer.[72][73][74][75][76] For diagnosis, combination of TK1 immunoassay with other biomarkers may be especially valuable[77] while falls in TK1 concentration following therapy may provide prognostic information.[78]
Breast cancer
Breast cancer is the commonest cancer in women by incidence (about 25% of cancer cases in USA and Europe) and the second largest by mortality (about 15%). The reason for this difference is the advances during the last decennia in the treatment of breast cancer cases and, above all, the public awareness that has allowed earlier diagnosis. One contributing factor is the widespread use of mammography for early detection, self-examination is another.
Many tumor markers including TK1 are used for the follow-up and detection of recurrences in breast cancer patients.[37][79][80][81][82][83][84] Immunoassays may be more sensitive than enzyme activity assays for detecting the TK1 forms found in the serum of subjects with breast cancer.[44] For diagnosis, combination of TK1 assays with other biomarkers, e.g. CA 15-3, may be especially valuable.[44]
Prostate cancer
Among men, prostate cancer is by far the commonest cancer form, forming about 25% of the total cancer incidence among men in USA and Europe. The mortality is much lower than would be expected from the incidence, around 10% of the total cancer mortality of men in USA and Europe. A major reason for the lower mortality is that many prostate cancers grow slowly so that the patients do not die from this cancer but from other unrelated reasons.
In the management of prostate cancer, it is, therefore, very important to be able to discriminate between slowly and rapidly growing cancers. Thymidine kinase has been suggested as a supplement to PSA (Prostate Specific Antigen), the tumor marker most frequently used in prostate cancer. Whereas PSA is considered to give an indication of the tumor mass, thymidine kinase activity indicates the rate of proliferation and the markers thus supplement each other.[85][86][87][88]
Other solid tumors
TK1 elevations have also been reported in association with many types of solid tumors including:
kidney cancer,[89] bladder cancer,[90] gastric cancer,[91][92][93] liver cancer,[94] neurological cancers[95] and melanoma.[96] Ovarian, cervical and esophageal cancers.[97]
Non-malignant elevations
There are several non-malignant causes for elevation of thymidine kinase in serum including vitamin B12 deficiency, leading to pernicious anemia[98][99] viral infections (particularly by virus from the herpes group) [100][101][99] and wound healing after trauma and operation.
Thymidine kinase in domestic animals
There are also reports of the use of thymidine kinase as a tumor marker in domestic animals, in horse,[102] in dogs[34][103][104][105][106][107] in cats[108] and in cows.[109] Elevations in dogs with bacterial infections have also been reported.[110]
Thymidine kinase in tissue
Thymidine kinase has been determined in tissue samples after extraction of the tissue and a relation between the results and disease progression has been shown. However, no standard method for the extraction or for the assay has been developed and TK determination in extracts from cells and tissues have not been validated in relation to any specific clinical question, see however Arnér et al.[111] Romain et al.[112] and Alegre et al.[113]
In the studies referred to below the methods used and the way the results are reported are so different that comparisons between different studies are not possible.
The TK1 levels in fetal tissues during development are higher than those of the corresponding tissues later.[114][115][116][117]
Certain non-malignant diseases also give rise to dramatic elevation of TK values in cells and tissue: in peripheral lymphocytes during monocytosis[118] and in bone marrow during pernicious anemia.[119][120] As TK1 is present in cells during cell division, it is reasonable to assume that the TK activity in malignant tissue should be higher than in corresponding normal tissue. This is also confirmed in most studies: a higher TK activity is found in neoplastic than in normal tissue,[115][121][122][123] in brain tumors,[124] in hematological malignancies,[125] in cancer and polyps in colon,[126][127][128][129][130][131] in breast cancer,[132][133][134][135][136][137] in lung cancer,[138][139][140] in gastric cancers,[141] in ovarian cancer,[142] in mesotheliomas,[143] in melanomas,[144] in thyroid tumors[145][146] in leukemia[147] and in breast cancer.[148]
Therapy that influences the rate of cell proliferation influences the TK values correspondingly. Although most studies do not show this, it seems probable that differences between samples from healthy tissue and samples from tumor tissue primarily represents changes in the levels of TK1, since this enzyme is much more strongly coupled to cell proliferation than TK2.
A method has been developed for specific determination of TK2 in cell extracts using the substrate analogue 5-Bromovinyl 2'-deoxyuridine.[117]
Uses of thymidine kinase determinations
Tumor markers may be used for the following purposes
- Screening either for specific cancers or generally for malignant growth. Broad screening for all or most types of cancer was early suggested[149][150] but has since been shown not to be a realistic goal. Screening for specific cancer types or locations requires a level of specificity and sensitivity that for tumor markers has so far only been reached by PSA.[151] Thymidine kinase neither reaches the clinical sensitivity nor the clinical specificity to be useful for screening purposes, see however Huang et al.,[152] Xiang et al.[153] and Cao et al.[154]
- Monitoring of cancer survivors after treatment, detection of recurrent disease is the most common use of tumor markers including thymidine kinase, that is used as a standard methods for monitoring hematological disorders, particularly lymphoma, but is also studied for monitoring solid tumors.
- Diagnosis of specific tumor types. The tumor types that are of interest for thymidine kinase are diagnosed by other techniques than measurement of tumor markers.
- Confirmation of diagnosis to verify the characteristics such as size and aggressivity of a tumor and thereby to help in the evaluation of a suitable treatment schedule has been verified as a suitable application of thymidine kinase determination for several types of tumors. Thymidine kinase has been confirmed as a valuable tool to verify the aggressivity of both hematologic tumors (particularly non-Hodgkin's lymphoma) and prostate carcinoma.
- Staging: thymidine kinase has been suggested for inclusion in the staging criteria for non-Hodgkin's lymphoma [63]
- Prognosis: thymidine kinase has been shown to be an important prognostic parameter particularly in hematologic malignancies (lymphoma and leukemia).
- Verification of the effect of treatment is an important use of thymidine kinase. As this tumor marker reacts to the activity of the tumor rather than to the tumor mass it gives a very early indication of the effect of the treatment.
- A companion diagnostic is used to verify if the treatment is suited for the type or subtype of tumor particularly in personalized medicine. The strong coupling of TK1 expression to the cell cycle provides a special rationale for investigating thymidine kinase as marker of effect of inhibitors to cyclin-dependent kinases. These inhibitor compounds constitute promising new cancer therapies. Cyclin-dependent kinases promote transition through the cell cycle and cyclin-dependent kinase inhibitors are intended to stop the transition to the S phase of the cell cycle, where thymidine kinase is synthesized. Serum TK-activity is therefore now included as a biomarker in clinical trials of these inhibitor compounds.[155]
See also
- Thymidine kinase
- Thymidylate kinase
- Nucleoside diphosphate kinase
- Thymidylate synthase
- Thymidine
Further reading
- O'Neill KL, Buckwalter M, Murray BK (2001). "Thymidine kinase: diagnostic and prognostic potential". Expert Rev. Mol. Diagn. 1 (4): 428–33. doi:10.1586/14737159.1.4.428. PMID 11901857.
- Topolcan O, Holubec Jr L (2008). "The role of thymidine kinase in cancer diseases". Expert Opin. Med. Diagn. 2 (2): 129–41. doi:10.1517/17530059.2.2.129. PMID 23485133.
- Jagarlamudi KK, Shaw M (2018). "Thymidine Kinase 1 as a Tumor Biomarker: Technical Advances offer New Potential to an Old Biomarker". Biomark. Med. 12 (9): 1035–48. doi:10.2217/bmm-2018-0157. PMID 30039979.
References
- Kit S (1985). "Thymidine kinase". Microbiol. Sci. 2 (12): 369–75. PMID 3939993.
- Wintersberger E (1997). "Regulation and biological function of thymidine kinase". Biochem. Soc. Trans. 25 (1): 303–8. doi:10.1042/bst0250303. PMID 9056888.
- Reichard P, Estborn B (1951). "Utilization of desoxyribosides in the synthesis of polynucleotides". J. Biol. Chem. 188 (2): 839–46. PMID 14824173.
- Bessman MJ, Kornberg A, Lehman IR, Simms ES (1956). "Enzymic synthesis of deoxyribonucleic acid". Biochim. Biophys. Acta. 21 (1): 197–8. doi:10.1016/0006-3002(56)90127-5. PMID 13363894.
- Bollum FJ, Potter VR (1958). "Incorporation of thymidine into deoxyribonucleic acid by enzymes from rat tissues". J. Biol. Chem. 233 (2): 478–82. PMID 13563524.
- Weissman SM, Smellie RM, Paul J (1960). "Studies on the biosynthesis of deoxyribonucleic acid by extracts of mammalian cells. IV. The phosphorylation of thymidine". Biochim. Biophys. Acta. 45: 101–10. doi:10.1016/0006-3002(60)91430-x. PMID 13784139.
- Gronowitz JS, Hagberg H, Källander CF, Simonsson B (1983). "The use of serum deoxythymidine kinase as a prognostic marker, and in the monitoring of patients with non-Hodgkin's lymphoma". Br. J. Cancer. 47 (4): 487–95. doi:10.1038/bjc.1983.78. PMC 2011337. PMID 6849793.
- Blasco R, López-Otín C, Muñóz M, Bockamp EO, Simón-Mateo C, Viñuela E (1990). "Sequence and evolutionary relationships of African swine fever virus thymidine kinase". Virology. 178 (1): 301–4. doi:10.1016/0042-6822(90)90409-k. PMID 2389555.
- Littlefield JW (1966). "The periodic synthesis of thymidine kinase in mouse fibroblasts". Biochim. Biophys. Acta. 114 (2): 398–403. doi:10.1016/0005-2787(66)90319-4. PMID 4223355.
- Berk AJ, Clayton DA (1973). "A genetically distinct thymidine kinase in mammalian mitochondria. Exclusive labeling of mitochondrial deoxyribonucleic acid". J. Biol. Chem. 248 (8): 2722–9. PMID 4735344.
- Berk AJ, Meyer BJ, Clayton DA (1973). "Mitochondrial-specific thymidine kinase". Arch. Biochem. Biophys. 154 (2): 563–5. doi:10.1016/0003-9861(73)90009-x. PMID 4632422.
- Zhu C, Harlow LS, Berenstein D, Munch-Petersen S, Munch-Petersen B (2006). "Effect of C-terminal of human cytosolic thymidine kinase (TK1) on in vitro stability and enzymatic properties". Nucleosides Nucleotides Nucleic Acids. 25 (9–11): 1185–8. doi:10.1080/15257770600894436. PMID 17065087.
- Kit S, Dubbs DR (1963). "Acquisition of thymidine kinase activity by herpes simplex-infected mouse fibroblast cells". Biochem. Biophys. Res. Commun. 11: 55–9. doi:10.1016/0006-291x(63)90027-5. PMID 14033128.
- McKnight SL (1980). "The nucleotide sequence and transcript map of the herpes simplex virus thymidine kinase gene". Nucleic Acids Res. 8 (24): 5949–64. doi:10.1093/nar/8.24.5949. PMC 328064. PMID 6258156.
- Halliburton IW, Morse LS, Roizman B, Quinn KE (1980). "Mapping of the thymidine kinase genes of type 1 and type 2 herpes simplex viruses using intertypic recombinants". J. Gen. Virol. 49 (2): 235–53. doi:10.1099/0022-1317-49-2-235. PMID 6255066.
- McDougall JK, Masse TH, Galloway DA (1980). "Location and cloning of the herpes simplex virus type 2 thymidine kinase gene". J. Virol. 33 (3): 1221–4. doi:10.1128/JVI.33.3.1221-1224.1980. PMC 288658. PMID 6245273.
- Kit S, Kit M, Qavi H, Trkula D, Otsuka H (1983). "Nucleotide sequence of the herpes simplex virus type 2 (HSV-2) thymidine kinase gene and predicted amino acid sequence of thymidine kinase polypeptide and its comparison with the HSV-1 thymidine kinase gene". Biochim. Biophys. Acta. 741 (2): 158–70. doi:10.1016/0167-4781(83)90056-8. PMID 6317035.
- Sawyer MH, Ostrove JM, Felser JM, Straus SE (1986). "Mapping of the varicella zoster virus deoxypyrimidine kinase gene and preliminary identification of its transcript". Virology. 149 (1): 1–9. doi:10.1016/0042-6822(86)90081-4. PMID 3004022.
- Littler E, Zeuthen J, McBride AA, Trøst Sørensen E, Powell KL, Walsh-Arrand JE, Arrand JR (1986). "Identification of an Epstein-Barr virus-coded thymidine kinase". EMBO J. 5 (8): 1959–66. doi:10.1002/j.1460-2075.1986.tb04450.x. PMC 1167064. PMID 3019675.
- Schlosser CA, Steglich C, deWet JR, Scheffler IE (1981). "Cell cycle-dependent regulation of thymidine kinase activity introduced into mouse LMTK- cells by DNA and chromatin-mediated gene transfer". Proc. Natl. Acad. Sci. U.S.A. 78 (2): 1119–23. Bibcode:1981PNAS...78.1119S. doi:10.1073/pnas.78.2.1119. PMC 319958. PMID 6940130.
- Coppock DL, Pardee AB (1987). "Control of thymidine kinase mRNA during the cell cycle". Mol. Cell. Biol. 7 (8): 2925–32. doi:10.1128/MCB.7.8.2925. PMC 367911. PMID 3670299.
- Stewart CJ, Ito M, Conrad SE (1987). "Evidence for transcriptional and post-transcriptional control of the cellular thymidine kinase gene". Mol. Cell. Biol. 7 (3): 1156–63. doi:10.1128/MCB.7.3.1156. PMC 365188. PMID 3561412.
- Piper AA, Tattersall MH, Fox RM (1980). "The activities of thymidine metabolising enzymes during the cell cycle of a human lymphocyte cell line LAZ-007 synchronised by centrifugal elutriation". Biochim. Biophys. Acta. 633 (3): 400–9. doi:10.1016/0304-4165(80)90198-1. PMID 6260157.
- Pelka-Fleischer R, Ruppelt W, Wilmanns W, Sauer H, Schalhorn A (1987). "Relation between cell cycle stage and the activity of DNA-synthesizing enzymes in cultured human lymphoblasts: investigations on cell fractions enriched according to cell cycle stages by way of centrifugal elutriation". Leukemia. 1 (3): 182–7. PMID 3669741.
- Sherley JL, Kelly TJ (1988). "Regulation of human thymidine kinase during the cell cycle". J. Biol. Chem. 263 (17): 8350–8. PMID 3372530.
- Gross MK, Kainz MS, Merrill GF (1987). "The chicken thymidine kinase gene is transcriptionally repressed during terminal differentiation: the associated decline in TK mRNA cannot account fully for the disappearance of TK enzyme activity". Dev. Biol. 122 (2): 439–51. doi:10.1016/0012-1606(87)90308-3. PMID 3596017.
- Kauffman MG, Kelly TJ (1991). "Cell cycle regulation of thymidine kinase: residues near the carboxyl terminus are essential for the specific degradation of the enzyme at mitosis". Mol. Cell. Biol. 11 (5): 2538–46. doi:10.1128/MCB.11.5.2538. PMC 360023. PMID 1708095.
- Sutterluety H, Bartl S, Karlseder J, Wintersberger E, Seiser C (1996). "Carboxy-terminal residues of mouse thymidine kinase are essential for rapid degradation in quiescent cells". J. Mol. Biol. 259 (3): 383–92. doi:10.1006/jmbi.1996.0327. PMID 8676376.
- Welin M, Kosinska U, Mikkelsen NE, Carnrot C, Zhu C, Wang L, Eriksson S, Munch-Petersen B, Eklund H (2004). "Structures of thymidine kinase 1 of human and mycoplasmic origin". Proc. Natl. Acad. Sci. U.S.A. 101 (52): 17970–5. Bibcode:2004PNAS..10117970W. doi:10.1073/pnas.0406332102. PMC 539776. PMID 15611477.
- Karlström AR, Neumüller M, Gronowitz JS, Källander CF (1990). "Molecular forms in human serum of enzymes synthesizing DNA precursors and DNA". Mol. Cell. Biochem. 92 (1): 23–35. doi:10.1007/BF00220716. PMID 2155379.
- Gronowitz JS, Källander CF (1980). "Optimized assay for thymidine kinase and its application to the detection of antibodies against herpes simplex virus type 1- and 2-induced thymidine kinase". Infection and Immunity. 29 (2): 425–34. PMC 551136. PMID 6260651.
- Gronowitz JS, Källander FR, Diderholm H, Hagberg H, Pettersson U (1984). "Application of an in vitro assay for serum thymidine kinase: results on viral disease and malignancies in humans". International Journal of Cancer. 33 (1): 5–12. doi:10.1002/ijc.2910330103. PMID 6693195.
- Gronowitz JS, Källander CF (1983). "A sensitive assay for detection of deoxythymidine kinase and its application to herpesvirus diagnosis". Current Topics in Microbiology and Immunology. 104: 235–45. doi:10.1007/978-3-642-68949-9_14. ISBN 978-3-642-68951-2. PMID 6307593.
- von Euler HP, Öhrvik AB, Eriksson SK (2006). "A non-radiometric method for measuring serum thymidine kinase activity in malignant lymphoma in dogs". Research in Veterinary Science. 80 (1): 17–24. doi:10.1016/j.rvsc.2005.05.001. PMID 16140350.
- Öhrvik A, Lindh M, Einarsson R, Grassi J, Eriksson S (2004). "Sensitive nonradiometric method for determining thymidine kinase 1 activity". Clinical Chemistry. 50 (9): 1597–606. doi:10.1373/clinchem.2003.030379. PMID 15247154.
- WO application 2006000246, "A method and kit for determination of thymidine kinase activity and use thereof", published 2006-02-24, assigned to Gronowitz JS
- Nisman B, Allweis T, Kadouri L, Mali B, Hamburger T, Baras M, Gronowitz S, Peretz T (2013). "Comparison of diagnostic and prognostic performance of two assays measuring thymidine kinase 1 activity in serum of breast cancer patients". Clin. Chem. Lab. Med. 51 (2): 439–47. doi:10.1515/cclm-2012-0162. PMID 23093267.
- Bacovsky J, Myslivecek M, Minarik J, Scudla V, Pika T, Zapletalova J, Petrova P, Bartkova M, Adam T, Gronowitz SJ (2015). "Analysis of thymidine kinase serum levels by novel method DiviTum in multiple myeloma and monoclonal gammopathy of undetermined significance - comparison with imaging methods 99mTc-MIBI scintigraphy and 18F-FDG PET/CT". Biomedical Papers of the Medical Faculty of the University Palacký, Olomouc, Czechoslovakia. 159 (1): 135–8. doi:10.5507/bp.2014.008. PMID 24572488.
- Stålhandske P, Wang L, Westberg S, von Euler H, Groth E, Gustafsson SA, Eriksson S, Lennerstrand J (2013). "Homogeneous assay for real-time and simultaneous detection of thymidine kinase 1 and deoxycytidine kinase activities". Anal. Biochem. 432 (2): 155–64. doi:10.1016/j.ab.2012.08.004. PMID 22902741.
- Alegre, MM; Weyant, MJ; Bennett, DT; Yu, JA; Ramsden, MK; Elnaggar, A; Robison, RA; O'Neill, KL (May 2014). "Serum detection of thymidine kinase 1 as a means of early detection of lung cancer". Anticancer Research. 34 (5): 2145–51. PMID 24778016.
- He, Q; Zhang, P; Zou, L; Li, H; Wang, X; Zhou, S; Fornander, T; Skog, S (October 2005). "Concentration of thymidine kinase 1 in serum (S-TK1) is a more sensitive proliferation marker in human solid tumors than its activity". Oncology Reports. 14 (4): 1013–9. PMID 16142366.
- Jagarlamudi, KK; Hansson, LO; Eriksson, S (18 February 2015). "Breast and prostate cancer patients differ significantly in their serum Thymidine kinase 1 (TK1) specific activities compared with those hematological malignancies and blood donors: implications of using serum TK1 as a biomarker". BMC Cancer. 15: 66. doi:10.1186/s12885-015-1073-8. PMC 4336758. PMID 25881026.
- Jagarlamudi KK, Hansson LO, Eriksson S (2015). "Breast and prostate cancer patients differ significantly in their serum Thymidine kinase 1 (TK1) specific activities compared with those hematological malignancies and blood donors: implications of using serum TK1 as a biomarker". BMC Cancer. 15: 66. doi:10.1186/s12885-015-1073-8. PMC 4336758. PMID 25881026.
- Kumar, JK; Aronsson, AC; Pilko, G; Zupan, M; Kumer, K; Fabjan, T; Osredkar, J; Eriksson, S (September 2016). "A clinical evaluation of the TK 210 ELISA in sera from breast cancer patients demonstrates high sensitivity and specificity in all stages of disease". Tumour Biology. 37 (9): 11937–11945. doi:10.1007/s13277-016-5024-z. PMC 5080325. PMID 27079872.
- He, Q; Skog, S; Wang, N; Eriksson, S; Tribukait, B (June 1996). "Characterization of a peptide antibody against a C-terminal part of human and mouse cytosolic thymidine kinase, which is a marker for cell proliferation". European Journal of Cell Biology. 70 (2): 117–24. PMID 8793383.
- He Q, Zou L, Zhang PA, Lui JX, Skog S, Fornander T (2000). "The clinical significance of thymidine kinase 1 measurement in serum of breast cancer patients using anti-TK1 antibody". The International Journal of Biological Markers. 15 (2): 139–46. doi:10.1177/172460080001500203. PMID 10883887.
- Pagaduan JV, Ramsden M, O'Neill K, Woolley AT (2015). "Microchip immunoaffinity electrophoresis of antibody-thymidine kinase 1 complex". Electrophoresis. 36 (5): 813–7. doi:10.1002/elps.201400436. PMC 4346389. PMID 25486911.
- Doi S, Naito K, Yamada K (1990). "Serum deoxythymidine kinase as a progressive marker of hematological malignancy". Nagoya J Med Sci. 52 (1–4): 19–26. PMID 2381458.
- Ellims PH, Van der Weyden MB, Medley G (1981). "Thymidine kinase isoenzymes in human malignant lymphoma". Cancer Res. 41 (2): 691–5. PMID 7448815.
- Hagberg H, Glimelius B, Gronowitz S, Killander A, Källander C, Schröder T (1984). "Biochemical markers in non-Hodgkin's lymphoma stages III and IV and prognosis: a multivariate analysis". Scand J Haematol. 33 (1): 59–67. doi:10.1111/j.1600-0609.1984.tb02211.x. PMID 6379852.
- Hallek M, Wanders L, Strohmeyer S, Emmerich B (1992). "Thymidine kinase: a tumor marker with prognostic value for non-Hodgkin's lymphoma and a broad range of potential clinical applications". Ann. Hematol. 65 (1): 1–5. doi:10.1007/bf01715117. PMID 1643153.
- Bogni A, Cortinois A, Grasselli G, Seregni E, Crippa F, Castellani MR, Bombardieri E (1994). "Thymidine kinase (TK) activity as a prognostic parameter of survival in lymphoma patients". J. Biol. Regul. Homeost. Agents. 8 (4): 121–5. PMID 7660854.
- Rehn S, Gronowitz JS, Källander C, Sundström C, Glimelius B (1995). "Deoxythymidine kinase in the tumor cells and serum of patients with non-Hodgkin lymphomas". Br. J. Cancer. 71 (5): 1099–105. doi:10.1038/bjc.1995.213. PMC 2033808. PMID 7734308.
- Suki S, Swan F, Tucker S, Fritsche HA, Redman JR, Rodriguez MA, McLaughlin P, Romaguera J, Hagemeister FB, Velasquez WS (1995). "Risk classification for large cell lymphoma using lactate dehydrogenase, beta-2 microglobulin, and thymidine kinase". Leukemia & Lymphoma. 18 (1–2): 87–92. doi:10.3109/10428199509064927. PMID 8580834.
- Suzuki K, Terui Y, Nakano K, Nara E, Nasu K, Ueda K, Nishimura N, Mishima Y, Sakajiri S, Yokoyama M, Takahashi S, Hatake K (2012). "High thymidine kinase activity is a strong predictive factor for poor prognosis in peripheral T-cell lymphoma treated with cyclophosphamide, adriamycin, vincristine and prednisone". Leukemia & Lymphoma. 53 (5): 849–54. doi:10.3109/10428194.2011.635858. PMID 22035416.
- Procházka V, Faber E, Raida L, Langová K, Indrák K, Papajík T (2012). "High baseline serum thymidine kinase 1 level predicts unfavorable outcome in patients with follicular lymphoma". Leukemia & Lymphoma. 53 (7): 1306–10. doi:10.3109/10428194.2011.654339. PMID 22263569.
- Suzuki K, Terui Y, Yokoyama M, Ueda K, Nishimura N, Mishima Y, Sakajiri S, Tsuyama N, Takeuchi K, Hatake K (2013). "Prognostic value of high thymidine kinase activity in patients with previously untreated diffuse large B-cell lymphoma treated by rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone". Leukemia & Lymphoma. 54 (11): 2412–7. doi:10.3109/10428194.2013.779690. PMID 23488601.
- Tsuji T, Satoh K, Nakano H, Nishide Y, Uemura Y, Tanaka S, Kogo M (2015). "Predictors of the necessity for lymph node biopsy of cervical lymphadenopathy". J Craniomaxillofac Surg. 43 (10): 2200–4. doi:10.1016/j.jcms.2015.09.010. PMID 26545929.
- Gatt ME, Goldschmidt N, Kalichman I, Friedman M, Aronson AC, Barak V (2015). "Thymidine kinase levels correlate with prognosis in aggressive lymphoma and can discriminate patients with a clinical suspicion of indolent to aggressive transformation". Anticancer Research. 35 (5): 3019–26. PMID 25964590.
- Källander CF, Simonsson B, Hagberg H, Gronowitz JS (1984). "Serum deoxythymidine kinase gives prognostic information in chronic lymphocytic leukemia". Cancer. 54 (11): 2450–5. doi:10.1002/1097-0142(19841201)54:11<2450::aid-cncr2820541123>3.0.co;2-r. PMID 6498737.
- Källander CF, Simonsson B, Gronowitz JS, Nilsson K (1987). "Serum deoxythymidine kinase correlates with peripheral lymphocyte thymidine uptake in chronic lymphocytic leukemia". Eur. J. Haematol. 38 (4): 331–7. doi:10.1111/j.1600-0609.1987.tb00007.x. PMID 3609253.
- Hallek M, Wanders L, Ostwald M, Busch R, Senekowitsch R, Stern S, Schick HD, Kuhn-Hallek I, Emmerich B (1996). "Serum beta(2)-microglobulin and serum thymidine kinase are independent predictors of progression-free survival in chronic lymphocytic leukemia and immunocytoma". Leukemia & Lymphoma. 22 (5–6): 439–47. doi:10.3109/10428199609054782. PMID 8882957.
- Rivkina A, Vitols G, Murovska M, Lejniece S (2011). "Identifying the stage of new CLL patients using TK, ZAP-70, CD38 levels". Experimental Oncology. 33 (2): 99–103. PMID 21716207.
- Bazargan A, Tam CS, Keating MJ (2012). "Predicting survival in chronic lymphocytic leukemia". Expert Review of Anticancer Therapy. 12 (3): 393–403. doi:10.1586/era.12.2. PMID 22369330.
- Pflug N, Bahlo J, Shanafelt TD, Eichhorst BF, Bergmann MA, Elter T, Bauer K, Malchau G, Rabe KG, Stilgenbauer S, Döhner H, Jäger U, Eckart MJ, Hopfinger G, Busch R, Fink AM, Wendtner CM, Fischer K, Kay NE, Hallek M (2014). "Development of a comprehensive prognostic index for patients with chronic lymphocytic leukemia". Blood. 124 (1): 49–62. doi:10.1182/blood-2014-02-556399. PMC 4260976. PMID 24797299.
- Hagag AA, Saad MA, Mohamed SA (2015). "Clinical significance of thymidine kinase in Egyptian children with acute lymphoblastic leukemia". South Asian Journal of Cancer. 4 (2): 72–4. doi:10.4103/2278-330X.155675. PMC 4418086. PMID 25992345.
- López-Martínez B, Vilchis Ordoñez A, Salazar Garcia M, Klünder-Klünder M, Parra-Ortega I, Dorantes-Acosta E, Angeles-Floriano T (2015). "Thymidine Kinase: A Biomarker for Recently Diagnosed Acute Leukemia in Pediatric Patients According to the Cell Line Involved". Arch. Med. Res. 46 (8): 630–4. doi:10.1016/j.arcmed.2015.11.005. PMID 26656666.
- Simonsson B, Källander CF, Brenning G, Killander A, Ahre A, Gronowitz JS (1985). "Evaluation of serum deoxythymidine kinase as a marker in multiple myeloma". British Journal of Haematology. 61 (2): 215–24. doi:10.1111/j.1365-2141.1985.tb02820.x. PMID 4041368.
- Simonsson B, Källander CF, Brenning G, Killander A, Gronowitz JS, Bergström R (1988). "Biochemical markers in multiple myeloma: a multivariate analysis". British Journal of Haematology. 69 (1): 47–53. doi:10.1111/j.1365-2141.1988.tb07601.x. PMID 3289607.
- Musto P, Bodenizza C, Falcone A, D'Arena G, Scalzulli P, Perla G, Modoni S, Parlatore L, Valvano MR, Carotenuto M (1995). "Prognostic relevance of serum thymidine kinase in primary myelodysplastic syndromes: relationship to development of acute myeloid leukaemia". British Journal of Haematology. 90 (1): 125–30. doi:10.1111/j.1365-2141.1995.tb03390.x. PMID 7786774.
- Aul C, Germing U, Gattermann N, Söhngen D, Heyll A (1996). "[The prognostic significance of serum thymidine kinase in the myelodysplastic syndrome]". Deutsche Medizinische Wochenschrift (in German). 121 (37): 1113–8. doi:10.1055/s-2008-1043114. PMID 8925725.
- Gronowitz JS, Steinholtz L, Källander CF, Hagberg H, Bergh J (1986). "Serum deoxythymidine kinase in small cell carcinoma of the lung. Relation to clinical features, prognosis, and other biochemical markers". Cancer. 58 (1): 111–8. doi:10.1002/1097-0142(19860701)58:1<111::aid-cncr2820580120>3.0.co;2-k. PMID 3011236.
- Gronowitz JS, Bergström R, Nôu E, Påhlman S, Brodin O, Nilsson S, Källander CF (1990). "Clinical and serologic markers of stage and prognosis in small cell lung cancer. A multivariate analysis". Cancer. 66 (4): 722–32. doi:10.1002/1097-0142(19900815)66:4<722::aid-cncr2820660421>3.0.co;2-j. PMID 2167141.
- Korkmaz T, Seber S, Okutur K, Basaran G, Yumuk F, Dane F, Ones T, Polat O, Madenci OC, Demir G, Turhal NS (2013). "Serum thymidine kinase 1 levels correlates with FDG uptake and prognosis in patients with non small cell lung cancer". Biomarkers. 18 (1): 88–94. doi:10.3109/1354750X.2012.738250. PMID 23116493.
- Nisman B, Nechushtan H, Biran H, Gantz-Sorotsky H, Peled N, Gronowitz S, Peretz T (2014). "Serum thymidine kinase 1 activity in the prognosis and monitoring of chemotherapy in lung cancer patients: a brief report". Journal of Thoracic Oncology. 9 (10): 1568–72. doi:10.1097/JTO.0000000000000276. PMID 25521401.
- Alegre MM, Weyant MJ, Bennett DT, Yu JA, Ramsden MK, Elnaggar A, Robison RA, O'Neill KL (2014). "Serum detection of thymidine kinase 1 as a means of early detection of lung cancer". Anticancer Research. 34 (5): 2145–51. PMID 24778016.
- Jiang, ZF; Wang, M; Xu, JL (1 February 2018). "Thymidine kinase 1 combined with CEA, CYFRA21-1 and NSE improved its diagnostic value for lung cancer". Life Sciences. 194: 1–6. doi:10.1016/j.lfs.2017.12.020. PMID 29247745.
- Lou, X; Zhou, J; Ma, H; Xu, S; He, E; Skog, S; Wang, H (August 2017). "The Half-Life of Serum Thymidine Kinase 1 Concentration Is an Important Tool for Monitoring Surgical Response in Patients with Lung Cancer: A Meta-Analysis". Genetic Testing and Molecular Biomarkers. 21 (8): 471–478. doi:10.1089/gtmb.2017.0003. PMID 28817340.
- Chen F, Tang L, Xia T, He E, Hu G, Li Y, Zhang M, Zhou J, Eriksson S, Skog S (2013). "Serum thymidine kinase 1 levels predict cancer-free survival following neoadjuvant, surgical and adjuvant treatment of patients with locally advanced breast cancer". Molecular and Clinical Oncology. 1 (5): 894–902. doi:10.3892/mco.2013.149. PMC 3915673. PMID 24649267.
- Nisman B, Allweis T, Kaduri L, Maly B, Gronowitz S, Hamburger T, Peretz T (2010). "Serum thymidine kinase 1 activity in breast cancer". Cancer Biomarkers. 7 (2): 65–72. doi:10.3233/CBM-2010-0148. PMID 21178264.
- Huang ZH, Tian XS, Li R, Wang XM, Wen W, Guan H, Yang YJ (2012). "Elevated thymidine kinase 1 in serum following neoadjuvant chemotherapy predicts poor outcome for patients with locally advanced breast cancer". Experimental and Therapeutic Medicine. 3 (2): 331–335. doi:10.3892/etm.2011.395. PMC 3438657. PMID 22969891.
- Nisman B, Kadouri L, Allweis T, Maly B, Hamburger T, Gronowitz S, Peretz T (2013). "Increased proliferative background in healthy women with BRCA1/2 haploinsufficiency is associated with high risk for breast cancer". Cancer Epidemiol. Biomarkers Prev. 22 (11): 2110–5. doi:10.1158/1055-9965.EPI-13-0193. PMID 23966579.
- Bjöhle J, Bergqvist J, Gronowitz JS, Johansson H, Carlsson L, Einbeigi Z, Linderholm B, Loman N, Malmberg M, Söderberg M, Sundquist M, Walz TM, Fernö M, Bergh J, Hatschek T (2013). "Serum thymidine kinase activity compared with CA 15-3 in locally advanced and metastatic breast cancer within a randomized trial". Breast Cancer Research and Treatment. 139 (3): 751–8. doi:10.1007/s10549-013-2579-x. PMID 23736998.
- Bolayirli M, Papila C, Korkmaz GG, Papila B, Aydoğan F, Karataş A, Uzun H (2013). "Serum thymidine kinase 1 activity in solid tumor (breast and colorectal cancer) patients treated with adjuvant chemotherapy". Journal of Clinical Laboratory Analysis. 27 (3): 220–6. doi:10.1002/jcla.21587. PMC 6807516. PMID 23686779.
- Larson A, Fritjofsson A, Norlén BJ, Gronowitz JS, Ronquist G (1985). "Prostate specific acid phosphatase versus five other possible tumor markers: a comparative study in men with prostatic carcinoma". Scandinavian Journal of Clinical and Laboratory Investigation. Supplementum. 179: 81–8. PMID 2417306.
- Lewenhaupt A, Ekman P, Eneroth P, Nilsson B (1990). "Tumour markers as prognostic aids in prostatic carcinoma". British Journal of Urology. 66 (2): 182–7. doi:10.1111/j.1464-410x.1990.tb14900.x. PMID 1697204.
- Ekman P, Lewenhaupt A (1991). "Serum tumour markers in human prostatic carcinoma. The value of a marker panel for prognostic information". Acta Oncol. 30 (2): 173–5. doi:10.3109/02841869109092345. PMID 2029401.
- Letocha H, Eklöv S, Gronowitz S, Norlén BJ, Nilsson S (1996). "Deoxythymidine kinase in the staging of prostatic adenocarcinoma". The Prostate. 29 (1): 15–9. doi:10.1002/(SICI)1097-0045(199607)29:1<15::AID-PROS2>3.0.CO;2-H. PMID 8685050.
- Nisman B, Yutkin V, Nechushtan H, Gofrit ON, Peretz T, Gronowitz S, Pode D (2010). "Circulating tumor M2 pyruvate kinase and thymidine kinase 1 are potential predictors for disease recurrence in renal cell carcinoma after nephrectomy". Urology. 76 (2): 513.e1–6. doi:10.1016/j.urology.2010.04.034. PMID 20573390.
- Rausch S, Hennenlotter J, Teepe K, Kuehs U, Aufderklamm S, Bier S, Mischinger J, Gakis G, Stenzl A, Schwentner C, Todenhöfer T (2015). "Muscle-invasive bladder cancer is characterized by overexpression of thymidine kinase 1". Urologic Oncology. 33 (10): 426.e21–9. doi:10.1016/j.urolonc.2015.06.007. PMID 26231311.
- Liu, Y; Ling, Y; Qi, Q; Tang, Y; Xu, J; Tong, Z; Sheng, G; Yang, Q; Pan, Y (November 2011). "Changes in serum thymidine kinase 1 levels during chemotherapy correlate with objective response in patients with advanced gastric cancer". Experimental and Therapeutic Medicine. 2 (6): 1177–1181. doi:10.3892/etm.2011.338. PMC 3440839. PMID 22977640.
- Liu Y, Ling Y, Qi Q, Tang Y, Xu J, Tong Z, Sheng G, Yang Q, Pan Y (2011). "Changes in serum thymidine kinase 1 levels during chemotherapy correlate with objective response in patients with advanced gastric cancer". Experimental and Therapeutic Medicine. 2 (6): 1177–1181. doi:10.3892/etm.2011.338. PMC 3440839. PMID 22977640.
- Ji Y, Wu XB, Chen JY, Hu B, Zhu QK, Zhu XF, Zheng MF (2015). "Serum thymidine kinase 1 levels correlate with clinical characteristics of esophageal squamous cell carcinoma". Int J Clin Exp Med. 8 (8): 12850–7. PMC 4612885. PMID 26550200.
- Zhang SY, Lin BD, Li BR (2015). "Evaluation of the diagnostic value of alpha-l-fucosidase, alpha-fetoprotein and thymidine kinase 1 with ROC and logistic regression for hepatocellular carcinoma". FEBS Open Bio. 5: 240–4. doi:10.1016/j.fob.2015.03.010. PMC 4392066. PMID 25870783.
- Kolberg M, Høland M, Lind GE, Ågesen TH, Skotheim RI, Hall KS, Mandahl N, Smeland S, Mertens F, Davidson B, Lothe RA (2015). "Protein expression of BIRC5, TK1, and TOP2A in malignant peripheral nerve sheath tumours--A prognostic test after surgical resection". Molecular Oncology. 9 (6): 1129–39. doi:10.1016/j.molonc.2015.02.005. PMC 5528761. PMID 25769404.
- Wu BJ, Li WP, Qian C, Ding W, Zhou ZW, Jiang H (2013). "Increased serum level of thymidine kinase 1 correlates with metastatic site in patients with malignant melanoma". Tumour Biology. 34 (2): 643–8. doi:10.1007/s13277-012-0591-0. PMID 23179401.
- Wang, Y; Jiang, X; Dong, S; Shen, J; Yu, H; Zhou, J; Li, J; Ma, H; He, E; Skog, S (11 March 2016). "Serum TK1 is a more reliable marker than CEA and AFP for cancer screening in a study of 56,286 people". Cancer Biomarkers. 16 (4): 529–36. doi:10.3233/CBM-160594. PMID 27002755.
- Ellims PH, Hayman RJ, Van der Weyden MB (1979). "Expression of fetal thymidine kinase in human cobalamin or folate deficient lymphocytes". Biochemical and Biophysical Research Communications. 89 (1): 103–7. doi:10.1016/0006-291x(79)90949-5. PMID 475797.
- Neumüller M, Källander CF, Gronowitz JS (1989). "Detection and characteristics of DNA polymerase activity in serum from patients with malignant, viral, or B12-deficiency disease". Enzyme. 41 (1): 6–16. doi:10.1159/000469045. PMID 2543552.
- Källander CF, Gronowitz JS, Olding-Stenkvist E (1983). "Rapid diagnosis of varicella-zoster virus infection by detection of viral deoxythymidine kinase in serum and vesicle fluid". Journal of Clinical Microbiology. 17 (2): 280–7. doi:10.1128/JCM.17.2.280-287.1983. PMC 272623. PMID 6339548.
- Tufveson G, Tötterman TH, Källander CF, Hagström A, Gronowitz JS (1988). "Serum thymidine-kinase and cytomegalovirus-specific antibodies after renal transplantation". Transplantation Proceedings. 20 (3): 405–7. PMID 2837850.
- Larsdotter S, Nostell K, von Euler H (2015). "Serum thymidine kinase activity in clinically healthy and diseased horses: a potential marker for lymphoma". Veterinary Journal. 205 (2): 313–6. doi:10.1016/j.tvjl.2015.01.019. PMID 25744802.
- von Euler H, Einarsson R, Olsson U, Lagerstedt AS, Eriksson S (2004). "Serum thymidine kinase activity in dogs with malignant lymphoma: a potent marker for prognosis and monitoring the disease". Journal of Veterinary Internal Medicine. 18 (5): 696–702. doi:10.1111/j.1939-1676.2004.tb02608.x. PMID 15515587.
- Jagarlamudi KK, Westberg S, Rönnberg H, Eriksson S (2014). "Properties of cellular and serum forms of thymidine kinase 1 (TK1) in dogs with acute lymphocytic leukemia (ALL) and canine mammary tumors (CMTs): implications for TK1 as a proliferation biomarker". BMC Veterinary Research. 10: 228. doi:10.1186/s12917-014-0228-1. PMC 4195903. PMID 25293656.
- Selting KA, Sharp CR, Ringold R, Knouse J (2015). "Serum thymidine kinase 1 and C-reactive protein as biomarkers for screening clinically healthy dogs for occult disease". Veterinary and Comparative Oncology. 13 (4): 373–84. doi:10.1111/vco.12052. PMID 23859156.
- Elliott JW, Cripps P, Blackwood L (2013). "Thymidine kinase assay in canine lymphoma". Veterinary and Comparative Oncology. 11 (1): 1–13. doi:10.1111/j.1476-5829.2011.00296.x. PMID 22236202.
- Jagarlamudi KK, Moreau L, Westberg S, Rönnberg H, Eriksson S (2015). "A New Sandwich ELISA for Quantification of Thymidine Kinase 1 Protein Levels in Sera from Dogs with Different Malignancies Can Aid in Disease Management". PLOS ONE. 10 (9): e0137871. Bibcode:2015PLoSO..1037871J. doi:10.1371/journal.pone.0137871. PMC 4569288. PMID 26366881.
- Taylor SS, Dodkin S, Papasouliotis K, Evans H, Graham PA, Belshaw Z, Westberg S, von Euler HP (2013). "Serum thymidine kinase activity in clinically healthy and diseased cats: a potential biomarker for lymphoma". Journal of Feline Medicine and Surgery. 15 (2): 142–7. doi:10.1177/1098612X12463928. PMID 23076596.
- Tawfeeq MM, Miura S, Horiuchi N, Kobayashi Y, Furuoka H, Inokuma H (2013). "Utility of serum thymidine kinase activity measurements for cases of bovine leukosis with difficult clinical diagnoses". The Journal of Veterinary Medical Science. 75 (9): 1167–72. doi:10.1292/jvms.12-0572. PMID 23628971.
- Sharif H, Hagman R, Wang L, Eriksson S (2013). "Elevation of serum thymidine kinase 1 in a bacterial infection: canine pyometra". Theriogenology. 79 (1): 17–23. doi:10.1016/j.theriogenology.2012.09.002. PMID 23102844.
- Arnér ES, Spasokoukotskaja T, Eriksson S (1992). "Selective assays for thymidine kinase 1 and 2 and deoxycytidine kinase and their activities in extracts from human cells and tissues". Biochemical and Biophysical Research Communications. 188 (2): 712–8. doi:10.1016/0006-291x(92)91114-6. PMID 1359886.
- Romain S, Spyratos F, Guirou O, Deytieux S, Chinot O, Martin PM (1994). "Technical evaluation of thymidine kinase assay in cytosols from breast cancers. EORTC Receptor Study Group Report". European Journal of Cancer. 30A (14): 2163–5. doi:10.1016/0959-8049(94)00376-g. PMID 7857717.
- Alegre MM, Robison RA, O'Neill KL (2012). "Thymidine kinase 1 upregulation is an early event in breast tumor formation". J Oncol. 2012: 1–5. doi:10.1155/2012/575647. PMC 3388419. PMID 22778736.
- Machovich R, Greengard O (1972). "Thymidine kinase in rat tissues during growth and differentiation". Biochimica et Biophysica Acta (BBA) - General Subjects. 286 (2): 375–81. doi:10.1016/0304-4165(72)90273-5. PMID 4660462.
- Herzfeld A, Greengard O (1980). "Enzyme activities in human fetal and neoplastic tissues". Cancer. 46 (9): 2047–54. doi:10.1002/1097-0142(19801101)46:9<2047::aid-cncr2820460924>3.0.co;2-q. PMID 6253048.
- Herzfeld A, Raper SM, Gore I (1980). "The ontogeny of thymidine kinase in tissues of man and rat". Pediatric Research. 14 (12): 1304–10. doi:10.1203/00006450-198012000-00006. PMID 7208144.
- Wang L, Eriksson S (2008). "5-Bromovinyl 2'-deoxyuridine phosphorylation by mitochondrial and cytosolic thymidine kinase (TK2 and TK1) and its use in selective measurement of TK2 activity in crude extracts". Nucleosides, Nucleotides & Nucleic Acids. 27 (6): 858–62. doi:10.1080/15257770802146510. PMID 18600552.
- Schollenberger S, Taureck D, Wilmanns W (1972). "[Enzymes of thymidine and thymidylate metabolism in normal and pathological blood and bone marrow cells]". Blut (in German). 25 (5): 318–34. doi:10.1007/BF01631814. PMID 4508724.
- Nakao K, Fujioka S (1968). "Thymidine kinase activity in the human bone marrow from various blood diseases". Life Sciences. 7 (8): 395–9. doi:10.1016/0024-3205(68)90039-8. PMID 5649653.
- Wickramasinghe SN, Olsen I, Saunders JE (1975). "Thymidine kinase activity in human bone marrow cells". Scandinavian Journal of Haematology. 15 (2): 139–44. doi:10.1111/j.1600-0609.1975.tb01065.x. PMID 1059244.
- Gordon HL, Bardos TJ, Chmielewicz ZF, Ambrus JL (1968). "Comparative study of the thymidine kinase and thymidylate kinase activities and of the feedbach inhibition of thymidine kinase in normal and neoplastic human tissue". Cancer Research. 28 (10): 2068–77. PMID 5696936.
- Stafford MA, Jones OW (1972). "The presence of "fetal" thymidine kinase in human tumors". Biochimica et Biophysica Acta (BBA) - Nucleic Acids and Protein Synthesis. 277 (2): 439–42. doi:10.1016/0005-2787(72)90423-6. PMID 4672678.
- Maehara Y, Nakamura H, Nakane Y, Kawai K, Okamoto M, Nagayama S, Shirasaka T, Fujii S (1982). "Activities of various enzymes of pyrimidine nucleotide and DNA syntheses in normal and neoplastic human tissues". Gan. 73 (2): 289–98. PMID 6288502.
- Persson L, Gronowitz SJ, Källander CF (1986). "Thymidine kinase in extracts of human brain tumours". Acta Neurochirurgica. 80 (3–4): 123–7. doi:10.1007/bf01812286. PMID 3012969.
- Filanovskaia LI, Togo AV, Shcherbakova EG, Blinov MN (1994). "[Thymidine kinase activity in leukocytes from patients with chronic myeloid leukemia at various periods in the disease]". Voprosy Medit︠S︡Inskoĭ Khimii (in Russian). 40 (1): 29–32. PMID 8122406.
- Lipkin M, Deschner E, Troncale F (1970). "Cell differentiation and the development of colonic neoplasms". CA: A Cancer Journal for Clinicians. 20 (6): 386–90. doi:10.3322/canjclin.20.6.386. PMID 4992499.
- Lipkin M (1971). "Proliferation and differentiation of normal and neoplastic cells in the colon of man". Cancer. 28 (1): 38–40. doi:10.1002/1097-0142(197107)28:1<38::aid-cncr2820280108>3.0.co;2-w. PMID 5110642.
- Weber G, Lui MS, Takeda E, Denton JE (1980). "Enzymology of human colon tumors". Life Sciences. 27 (9): 793–9. doi:10.1016/0024-3205(80)90333-1. PMID 7412505.
- Sagara T, Tsukada K, Iwama T, Mishima Y, Sakamoto S, Okamoto R (1985). "[Thymidine kinase isozymes in human colon polyps]". Nihon Gan Chiryo Gakkai Shi (in Japanese). 20 (7): 1312–6. PMID 4078430.
- Sakamoto S, Sagara T, Iwama T, Kawasaki T, Okamoto R (1985). "Increased activities of thymidine kinase isozymes in human colon polyp and carcinoma". Carcinogenesis. 6 (6): 917–9. doi:10.1093/carcin/6.6.917. PMID 4006080.
- Sakamoto S, Okamoto R (1992). "Thymidine kinase activity in familial adenomatous polyposis". The Tohoku Journal of Experimental Medicine. 168 (2): 291–301. doi:10.1620/tjem.168.291. PMID 1339104.
- Sakamoto S, Iwama T, Ebuchi M, Tsukada K, Sagara T, Kawasaki T, Murakami S, Kasahara N, Kudo H, Okamoto R (1986). "Increased activities of thymidine kinase isozymes in human mammary tumours". The British Journal of Surgery. 73 (4): 272–3. doi:10.1002/bjs.1800730409. PMID 3697655.
- Galloux H, Javre JL, Guerin D, Sampérez S, Jouan P (1988). "[Prognostic value of fetal thymidine kinase measurements in breast cancer]". Comptes Rendus de l'Académie des Sciences, Série III (in French). 306 (3): 89–92. PMID 3126994.
- Romain S, Javre JL, Samperez S, Jouan P, Bressac C, Varette I, Brandone H, Martin PM (1990). "[Prognostic value of thymidine kinase in cancer of the breast]". Bulletin du Cancer (in French). 77 (10): 973–83. PMID 2249017.
- O'Neill KL, Hoper M, Odling-Smee GW (1992). "Can thymidine kinase levels in breast tumors predict disease recurrence?". Journal of the National Cancer Institute. 84 (23): 1825–8. doi:10.1093/jnci/84.23.1825. PMID 1433372.
- O'Neill KL, McKelvey VJ, Hoper M, Monteverde H, Odling-Smee GW, Logan H, Abram WP, McKenna PG (1992). "Breast tumour thymidine kinase levels and disease recurrence". Medical Laboratory Sciences. 49 (4): 244–7. PMID 1339926.
- Romain S, Chinot O, Guirou O, Soullière M, Martin PM (1994). "Biological heterogeneity of ER-positive breast cancers in the post-menopausal population". International Journal of Cancer. 59 (1): 17–9. doi:10.1002/ijc.2910590105. PMID 7927897.
- Greengard O, Head JF, Goldberg SL, Kirschner PA (1982). "Enzyme pathology and the histologic categorization of human lung tumors: the continuum of quantitative biochemical indices of neoplasticity". Cancer. 49 (3): 460–7. doi:10.1002/1097-0142(19820201)49:3<460::aid-cncr2820490312>3.0.co;2-y. PMID 6277448.
- Greengard O, Head JF, Goldberg SL, Kirschner PA (1985). "Biochemical measure of the volume doubling time of human pulmonary neoplasms". Cancer. 55 (7): 1530–5. doi:10.1002/1097-0142(19850401)55:7<1530::aid-cncr2820550720>3.0.co;2-v. PMID 2983858.
- Yusa T, Tamiya N, Yamaguchi Y, Takeda T, Ogawa T, Kimura H, Fujimura S (1994). "[A study of thymidine kinase activity in lung cancer tissue]". Nihon KyōBu Shikkan Gakkai Zasshi (in Japanese). 32 (3): 211–5. PMID 8189640.
- Konishi T, Miyama T, Sakamoto S, Hirata T, Mafune K, Hiraishi M, Idezuki Y (1992). "Activities of thymidylate synthetase and thymidine kinase in gastric cancer". Surgical Oncology. 1 (3): 215–21. doi:10.1016/0960-7404(92)90067-u. PMID 1341254.
- Look KY, Moore DH, Sutton GP, Prajda N, Abonyi M, Weber G (1997). "Increased thymidine kinase and thymidylate synthase activities in human epithelial ovarian carcinoma". Anticancer Research. 17 (4A): 2353–6. PMID 9252646.
- Greengard O, Head JF, Chahinian AP, Goldberg SL (1987). "Enzyme pathology of human mesotheliomas". Journal of the National Cancer Institute. 78 (4): 617–22. doi:10.1093/jnci/78.4.617. PMID 2882044.
- Borovanský J, Stríbrná J, Elleder M, Netíková I (1994). "Thymidine kinase in malignant melanoma". Melanoma Research. 4 (5): 275–9. doi:10.1097/00008390-199410000-00001. PMID 7858409.
- Sakamoto S, Murakami S, Sugawara M, Mishima Y, Okamoto R (1991). "Increased activities of thymidylate synthetase and thymidine kinase in human thyroid tumors". Thyroid. 1 (4): 347–51. doi:10.1089/thy.1991.1.347. PMID 1841732.
- Pikner R, Ludvíkova M, Ryska A, Kholova I, Holubec L, Topolcan O, Pecen L, Fínek J (2005). "TPS, thymidine kinase, VEGF and endostatin in cytosol of thyroid tissue samples". Anticancer Research. 25 (3A): 1517–21. PMID 16033053.
- Wilms K, Wilmanns W (1972). "[Effects of dauno-rubidomycin and adriamycin on enzymes of DNA synthesis in leukocytes in vivo and in culture]". Klinische Wochenschrift (in German). 50 (18): 866–70. doi:10.1007/bf01488943. PMID 4507472.
- Zhang HJ, Kennedy BJ, Kiang DT (1984). "Thymidine kinase as a predictor of response to chemotherapy in advanced breast cancer". Breast Cancer Research and Treatment. 4 (3): 221–5. doi:10.1007/bf01806488. PMID 6487823.
- Björklund B (1962). "Immunological approaches to the study of cancer". Rontgen Laborator. 15: L21–L28. PMID 13869604.
- Björklund B (1978). "Tissue polypeptide antigen (TPA): Biology, biochemistry, improved assay methodology, clinical significance in cancer and other conditions, and future outlook". Laboratory Testing for Cancer. Antibiotics and Chemotherapy. 22. pp. 16–31. doi:10.1159/000401148. ISBN 978-3-8055-2765-1. PMID 623439.
- Vickers AJ, Eastham JA, Scardino PT, Lilja H (2016). "The Memorial Sloan Kettering Cancer Center Recommendations for Prostate Cancer Screening". Urology. 91: 12–8. doi:10.1016/j.urology.2015.12.054. PMC 4842100. PMID 26850815.
- Huang S, Lin J, Guo N, Zhang M, Yun X, Liu S, Zhou J, He E, Skog S (2011). "Elevated serum thymidine kinase 1 predicts risk of pre/early cancerous progression". Asian Pacific Journal of Cancer Prevention. 12 (2): 497–505. PMID 21545220.
- Xiang Y, Zeng H, Liu X, Zhou H, Luo L, Duan C, Luo X, Yan H (2013). "Thymidine kinase 1 as a diagnostic tumor marker is of moderate value in cancer patients: A meta-analysis". Biomedical Reports. 1 (4): 629–637. doi:10.3892/br.2013.114. PMC 3916991. PMID 24648999.
- Cao X, Wang Y, Yang P, Zhou H, Liu C, Chen Z (2014). "[Application of serum thymidine kinase 1 of 26 055 cases in health screening for early detection of premalignant/early malignant tumors]". Zhong Nan da Xue Xue Bao. Yi Xue Ban = Journal of Central South University. Medical Sciences (in Chinese). 39 (10): 1029–34. doi:10.11817/j.issn.1672-7347.2014.10.007. PMID 25355255.
- Asghar U, Witkiewicz AK, Turner NC, Knudsen ES (2015). "The history and future of targeting cyclin-dependent kinases in cancer therapy". Nat Rev Drug Discov. 14 (2): 130–46. doi:10.1038/nrd4504. PMC 4480421. PMID 25633797.
External links
- Thymidine+kinase at the US National Library of Medicine Medical Subject Headings (MeSH)