1,8-Diazabicyclo(5.4.0)undec-7-ene
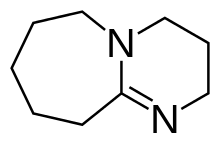
1,8-Diazabicyclo[5.4.0]undec-7-ene, or more commonly DBU, is a chemical compound and belongs to the class of amidine compounds. It is used in organic synthesis as a catalyst, a complexing ligand, and a non-nucleophilic base.[3]
 | |
 | |
| Names | |
|---|---|
| IUPAC name
2,3,4,6,7,8,9,10-octahydropyrimido[1,2-a]azepine | |
| Other names
DBU,Diazabicycloundecene | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.027.013 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C9H16N2 | |
| Molar mass | 152.241 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 1.018 g/mL liquid |
| Melting point | −70 °C (−94 °F; 203 K) |
| Boiling point | 80 to 83 °C (176 to 181 °F; 353 to 356 K) (0.6 mmHg); 261 °C (1 atm) |
| Acidity (pKa) | 13.5±1.5[1] (pKa of conjugate acid in water); 24.34[2] (pKa of conjugate acid in acetonitrile) |
| Hazards | |
| GHS pictograms |    |
| GHS Signal word | Danger |
GHS hazard statements |
H301, H302, H312, H314, H318, H412 |
| P260, P264, P270, P273, P280, P301+310, P301+312, P301+330+331, P302+352, P303+361+353, P304+340, P305+351+338, P310, P312, P321, P322, P330, P363, P405, P501 | |
| Flash point | 119.9 °C (247.8 °F; 393.0 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Occurrence
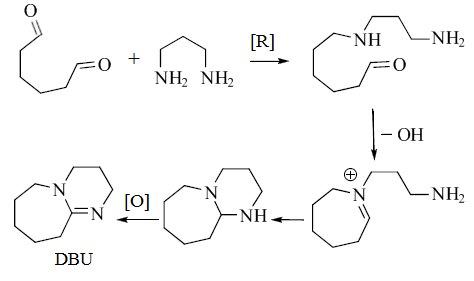
Although all commercially available DBU is produced synthetically, it can also be isolated from the sea sponge Niphates digitalis.[4] The biosynthesis of DBU has been proposed to begin with 1,6-hexanedial and 1,3-diaminopropane.[4]
Uses
As a reagent in organic chemistry, DBU is used as a catalyst, a complexing ligand, and a non-nucleophilic base. It is also used as a curing agent for epoxy. It is used in fullerene purification with trimethylbenzene (it reacts with C70 and higher fullerenes, but not to C60 fullerenes); and it is also used as a catalyst in polyurethane production. It has a strong catalyst effect for the reactions of alicyclic and aliphatic isocyanates. It also exhibited its dual character (base and nucleophile) in the synthesis of aryl- and styryl-terminal acetylenes.
See also
References
- Kaupmees, K.; Trummal, A.; Leito, I. (2014). "Basicities of Strong Bases in Water: A Computational Study". Croat. Chem. Acta. 87 (4): 385–395. doi:10.5562/cca2472.
- Kaljurand, I.; Kütt, A.; Sooväli, L.; Rodima, T.; Mäemets, V.; Leito, I.; Koppel, I. A. (2005). "Extension of the Self-Consistent Spectrophotometric Basicity Scale in Acetonitrile to a Full Span of 28 pKa Units: Unification of Different Basicity Scales". J. Org. Chem. 70 (3): 1019–1028. doi:10.1021/jo048252w. PMID 15675863.
- Ghosh, Nandita (2004). "DBU (1,8-diazabicyclo[5.4.0]undec-7-ene) - A Nucleophillic Base". Synlett (3): 574–575. doi:10.1055/s-2004-815436.
- Regalado, E.L. et al., Nat. Prod. Commun., 2010, 5, 1187- 1190