meta-Chloroperoxybenzoic acid
meta-Chloroperoxybenzoic acid (mCPBA or mCPBA) is a peroxycarboxylic acid. A white solid, it is used widely as an oxidant in organic synthesis. mCPBA is often preferred to other peroxy acids because of its relative ease of handling.[1] mCPBA is a strong oxidizing agent that may cause fire upon contact with flammable material.[2]
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
3-Chlorobenzene-1-carboperoxoic acid | |||
| Other names
3-Chloroperoxybenzoic acid 3-Chloroperbenzoic acid 3-Chlorobenzoperoxoic acid meta-Chloroperoxybenzoic acid m-Chloroperoxybenzoic acid meta-Chloroperbenzoic acid mCPBA m-CPBA | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChemSpider | |||
| ECHA InfoCard | 100.012.111 | ||
| EC Number |
| ||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 3106 | ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C7H5ClO3 | |||
| Molar mass | 172.56 g·mol−1 | ||
| Appearance | White powder | ||
| Melting point | 92 to 94 °C (198 to 201 °F; 365 to 367 K) decomposes | ||
| Acidity (pKa) | 7.57 | ||
| Hazards | |||
| Main hazards | Oxidizing, corrosive, explosive | ||
| GHS pictograms |    | ||
| GHS Signal word | Danger | ||
GHS hazard statements |
H226, H314, H318, H335 | ||
| P210, P220, P233, P234, P240, P241, P242, P243, P260, P261, P264, P271, P272, P280, P301+330+331, P302+352, P303+361+353, P304+340, P305+351+338, P310, P312, P321, P332+313, P333+313, P337+313 | |||
| Related compounds | |||
Related compounds |
peroxyacetic acid; peroxybenzoic acid | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Preparation and purification
mCPBA can be prepared by reacting m-Chlorobenzoyl chloride with a basic solution of hydrogen peroxide, followed by acidification.[3]
It is sold commercially as a shelf-stable mixture that is less than 72% mCPBA, with the balance made up of m-chlorobenzoic acid (10%) and water.[1] The peroxyacid can be purified by washing the commercial material with a solution buffered at pH = 7.5.[2][4] Peroxyacids are generally slightly less acidic than their carboxylic acid counterparts, so one can extract the acid impurity by careful control of pH. The purified material is reasonably stable against decomposition if stored at low temperatures in a plastic container.
In reactions where the exact amount of mCPBA must be controlled, a sample can be titrated to determine the exact amount of active oxidant.
Reactions
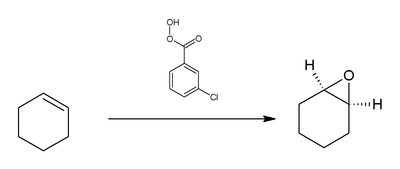
The main areas of use are the conversion of ketones to esters (Baeyer-Villiger oxidation), epoxidation of alkenes (Prilezhaev reaction), conversion of silyl enol ethers to silyl α-hydroxy ketones (Rubottom oxidation), oxidation of sulfides to sulfoxides and sulfones, and oxidation of amines to produce amine oxides. The following scheme shows the epoxidation of cyclohexene with mCPBA.
The epoxidation mechanism is concerted: the cis or trans geometry of the alkene starting material is retained in the epoxide ring of the product. The transition state of the Prilezhaev reaction is given below:
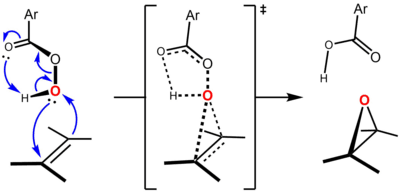
The geometry of the transition state, with the peracid bisecting the C-C double bond, allows the two primary frontier orbital interactions to occur: πC=C (HOMO) to σ*O-O (LUMO) and nO (HOMO, regarded as a filled p orbital on a sp2 hybridized oxygen) to π*C=C (LUMO), corresponding, in arrow-pushing terms, to formation of one C-O bond and cleavage of the O-O bond and formation of the other C-O bond and cleavage of the C=C π bond.
References
- "3-Chloroperoxybenzoic acid". Organic Chemistry Portal.
- Rao, A. Somasekar; Mohan, H. Rama; Charette, André (2005). "m‐Chloroperbenzoic Acid". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rc140.pub2. ISBN 0471936235.
- McDonald, Richard N.; Steppel, Richard N.; Dorsey, James E. (1970). "m-Chloroperbenzoic Acid". Organic Syntheses. 50: 15. doi:10.15227/orgsyn.050.0015.
- Armarego, W. L. F.; Perrin, D. D. (1996). Purification of Laboratory Chemicals (4th ed.). Oxford: Butterworth-Heinemann. p. 145. ISBN 0-7506-3761-7.