Pyritinol
Pyritinol also called pyridoxine disulfide or pyrithioxine (European drug names Encephabol, Encefabol, Cerbon 6) is a semi-synthetic water-soluble analog of vitamin B6 (Pyridoxine HCl). It was produced in 1961 by Merck Laboratories by bonding 2 vitamin B6 compounds (pyridoxine) together with a disulfide bridge. Since the 1970s, it has been a prescription and OTC drug in several countries for cognitive disorders, rheumatoid arthritis,[1] and learning disorders in children. Since the early 1990s it has been sold as a nootropic dietary supplement in the United States.
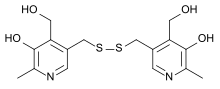 | |
| Clinical data | |
|---|---|
| ATC code | |
| Pharmacokinetic data | |
| Elimination half-life | 2.5 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.012.864 |
| Chemical and physical data | |
| Formula | C16H20N2O4S2 |
| Molar mass | 368.473 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Availability
It is approved for "symptomatic treatment of chronically impaired brain function in dementia syndromes" and for "supportive treatment of sequelae of craniocerebral trauma" in various European countries, including Austria, Germany, France, Italy, Portugal, and Greece. In France it is also approved for rheumatoid arthritis as a disease modifying drug, on the basis of the results of clinical trials. In many countries it is available over the counter and is widely advertised on the internet as being for "memory disturbances."
Effects
One small study, with 12 subjects given pyritinol, showed an improvement in performance on tests of reaction time, but not on memory tests.[2]
Some studies have found large doses of Pyritinol can help to reduce hangovers.[3]
Showed improvement over placebo in those with senile dementia of the Alzheimer type (SDAT) and multi-infarct dementia (MID).[4]
In healthy adults it improved several measure of cognition treating in one placebo controlled study.[5]
Adverse effects
Adverse effects include nausea, headache,[6] and rarely allergic reaction (mild skin reactions).[7] A 2004 survey of six case reports suggested a link between pyritinol and severe cholestatic hepatitis when on several drugs for certain diseases.[8]
Other rare side effects: acute pancreatitis[9] and photoallergic eruption.[10]
See also
References
- Lemmel EM (May 1993). "Comparison of pyritinol and auranofin in the treatment of rheumatoid arthritis. The European Multicentre Study Group". British Journal of Rheumatology. 32 (5): 375–82. doi:10.1093/rheumatology/32.5.375. PMID 8495257.
- Hindmarch I, Coleston DM, Kerr JS (1990). "Psychopharmacological effects of pyritinol in normal volunteers". Neuropsychobiology. 24 (3): 159–64. doi:10.1159/000119478. PMID 2135070.
- Wiese JG, Shlipak MG, Browner WS (June 2000). "The alcohol hangover". Annals of Internal Medicine. 132 (11): 897–902. doi:10.7326/0003-4819-132-11-200006060-00008. PMID 10836917.
- Fischhof PK, Saletu B, Rüther E, Litschauer G, Möslinger-Gehmayr R, Herrmann WM (1992). "Therapeutic efficacy of pyritinol in patients with senile dementia of the Alzheimer type (SDAT) and multi-infarct dementia (MID)". Neuropsychobiology. 26 (1–2): 65–70. doi:10.1159/000118898. PMID 1475039.
- Hindmarch I, Coleston DM, Kerr JS (1990). "Psychopharmacological effects of pyritinol in normal volunteers". Neuropsychobiology. 24 (3): 159–64. doi:10.1159/000119478. PMID 2135070.
- Nachbar F, Korting HC, Vogl T (1993). "Erythema multiforme-like eruption in association with severe headache following pyritinol". Dermatology. 187 (1): 42–6. doi:10.1159/000247196. PMID 8324277.
- de Groot, Anton C.; Nater, Johan Pieter; Weyland, J. Willem. Unwanted Effects of Cosmetics and Drugs Used in Dermatology.
- Maria V, Albuquerque A, Loureiro A, Sousa A, Victorino R (March 2004). "Severe cholestatic hepatitis induced by pyritinol". BMJ. 328 (7439): 572–4. doi:10.1136/bmj.328.7439.572. PMC 381054. PMID 15001508.
- Straumann A, Bauer M, Pichler WJ, Pirovino M (August 1998). "Acute pancreatitis due to pyritinol: an immune-mediated phenomenon". Gastroenterology. 115 (2): 452–4. doi:10.1016/S0016-5085(98)70212-4. PMID 9679051.
- Tanaka M, Niizeki H, Shimizu S, Miyakawa S (October 1996). "Photoallergic drug eruption due to pyridoxine hydrochloride". The Journal of Dermatology. 23 (10): 708–9. doi:10.1111/j.1346-8138.1996.tb02685.x. PMID 8973037.
External links
