Plastic
Plastics are a wide range of synthetic or semi-synthetic organic compounds that are malleable and so can be molded into solid objects.

Plasticity is the general property of all materials which can deform irreversibly without breaking but, in the class of moldable polymers, this occurs to such a degree that their actual name derives from this specific ability.
Plastics are typically organic polymers of high molecular mass and often contain other substances. They are usually synthetic, most commonly derived from petrochemicals, however, an array of variants are made from renewable materials such as polylactic acid from corn or cellulosics from cotton linters.[1]
In developed economies, about a third of plastic is used in packaging and roughly the same in buildings in applications such as piping, plumbing or vinyl siding.[2] Other uses include automobiles (up to 20% plastic[2]), furniture, and toys.[2] In the developing world, the applications of plastic may differ—42% of India's consumption is used in packaging.[2] Worldwide, about 50 kg of plastic is produced annually per person, with production doubling every ten years.
Plastics have many uses in the medical field as well, with the introduction of polymer implants and other medical devices derived at least partially from plastic. The field of plastic surgery is not named for use of plastic materials, but rather the meaning of the word plasticity, with regard to the reshaping of flesh.
The world's first fully synthetic plastic was bakelite, invented in New York in 1907, by Leo Baekeland[3] who coined the term 'plastics'.[4] Many chemists have contributed to the materials science of plastics, including Nobel laureate Hermann Staudinger who has been called "the father of polymer chemistry" and Herman Mark, known as "the father of polymer physics".[5]
The success and dominance of plastics starting in the early 20th century led to environmental concerns regarding its slow decomposition rate after being discarded as trash due to its composition of large molecules. Toward the end of the century, one approach to this problem was met with wide efforts toward recycling.
Etymology
The word plastic derives from the Greek πλαστικός (plastikos) meaning "capable of being shaped or molded" and, in turn, from πλαστός (plastos) meaning "molded".[6][7]
The plasticity, or malleability, of the material during manufacture allows it to be cast, pressed, or extruded into a variety of shapes, such as: films, fibers, plates, tubes, bottles, boxes, amongst many others.
The common noun plastic should not be confused with the technical adjective plastic. The adjective is applicable to any material which undergoes a plastic deformation, or permanent change of shape, when strained beyond a certain point. For example, aluminum which is stamped or forged exhibits plasticity in this sense, but is not plastic in the common sense. By contrast, some plastics will, in their finished forms, break before deforming and therefore are not plastic in the technical sense.
Structure
Most plastics contain organic polymers.[8] The vast majority of these polymers are formed from chains of carbon atoms, 'pure' or with the addition of: oxygen, nitrogen, or sulfur. The chains comprise many repeat units, formed from monomers. Each polymer chain will have several thousand repeating units.
The backbone is the part of the chain that is on the "main path", linking together a large number of repeat units.
To customize the properties of a plastic, different molecular groups "hang" from this backbone. These pendant units are usually "hung" on the monomers, before the monomers themselves are linked together to form the polymer chain. It is the structure of these side chains that influences the properties of the polymer.
The molecular structure of the repeating unit can be fine tuned to influence specific properties of the polymer.
Properties and classifications
Plastics are usually classified by: the chemical structure of the polymer's backbone and side chains; some important groups in these classifications are: the acrylics, polyesters, silicones, polyurethanes, and halogenated plastics.
Plastics can also be classified by: the chemical process used in their synthesis, such as: condensation, polyaddition, and cross-linking.[9]
Plastics can also be classified by: their various physical properties, such as: hardness, density, tensile strength, resistance to heat and glass transition temperature, and by their chemical properties, such as the organic chemistry of the polymer and its resistance and reaction to various chemical products and processes, such as: organic solvents, oxidation, and ionizing radiation. In particular, most plastics will melt upon heating to a few hundred degrees celsius.[10]
Other classifications are based on qualities that are relevant for manufacturing or product design. Examples of such qualities and classes are: thermoplastics and thermosets, conductive polymers, biodegradable plastics and engineering plastics and other plastics with particular structures, such as elastomers.
Thermoplastics and thermosetting polymers

One important classification of plastics is by the permanence or impermanence of their form, or whether they are: thermoplastics or thermosetting polymers. Thermoplastics are the plastics that, when heated, do not undergo chemical change in their composition and so can be molded again and again. Examples include: polyethylene (PE), polypropylene (PP), polystyrene (PS) and polyvinyl chloride (PVC).[11] Common thermoplastics range from 20,000 to 500,000 amu, while thermosets are assumed to have infinite molecular weight.
Thermosets, or thermosetting polymers, can melt and take shape only once: after they have solidified, they stay solid.[12] In the thermosetting process, a chemical reaction occurs that is irreversible. The vulcanization of rubber is an example of a thermosetting process: before heating with sulfur, the polyisoprene is a tacky, slightly runny material; after vulcanization, the product is rigid and non-tacky.
Amorphous plastics and crystalline plastics
Many plastics are completely amorphous,[13] such as: all thermosets; polystyrene and its copolymers; and polymethyl methacrylate.
However, some plastics are partially crystalline and partially amorphous in molecular structure, giving them both a melting point, the temperature at which the attractive intermolecular forces are overcome, and also one or more glass transitions, the temperatures above which the extent of localized molecular flexibility is substantially increased. These so-called semi-crystalline plastics include: polyethylene, polypropylene, polyvinyl chloride, polyamides (nylons), polyesters and some polyurethanes.
Conductive polymers
Intrinsically Conducting Polymers (ICP) are organic polymers that conduct electricity. While plastics can be made electrically conductive, with a conductivity of up to 80 kS/cm in stretch-oriented polyacetylene,[14] they are still no match for most metals like copper which have a conductivity of several hundred kS/cm. Nevertheless, this is a developing field.
Biodegradable plastics and bioplastics
Biodegradable plastics are plastics that degrade, or break down, upon exposure to: sunlight or ultra-violet radiation, water or dampness, bacteria, enzymes or wind abrasion. In some instances, rodent, pest, or insect attack can also be considered as forms of biodegradation or environmental degradation.
Some modes of degradation require that the plastic be exposed at the surface (aerobic), whereas other modes will only be effective if certain conditions exist in landfill or composting systems (anaerobic).
Some companies produce biodegradable additives, to enhance biodegradation. Plastic can have starch powder added as a filler to allow it to degrade more easily, but this still does not lead to the complete breaking down of the plastic.
Some researchers have genetically engineered bacteria to synthesize completely biodegradable plastics, such as Biopol; however, these are expensive at present.[15]
Bioplastics
While most plastics are produced from petrochemicals, bioplastics are made substantially from renewable plant materials such: as cellulose and starch.[16] Due both to the finite limits of the petrochemical reserves and to the threat of global warming, the development of bioplastics is a growing field.
However, bioplastic development begins from a very low base and, as yet, does not compare significantly with petrochemical production. Estimates of the global production capacity for bio-derived materials is put at 327,000 tonnes/year. In contrast, global production of polyethylene (PE) and polypropylene (PP), the world's leading petrochemical derived polyolefins, was estimated at over 150 million tonnes in 2015.[17]
Types
Common plastics


This category includes both commodity plastics, or standard plastics, and engineering plastics.
- Polyamides (PA) or (nylons) – fibers, toothbrush bristles, tubing, fishing line and low-strength machine parts such as engine parts or gun frames
- Polycarbonate (PC) – compact discs, eyeglasses, riot shields, security windows, traffic lights and lenses
- Polyester (PES) – fibers and textiles
- Polyethylene (PE) – a wide range of inexpensive uses including supermarket bags and plastic bottles
- High-density polyethylene (HDPE) – detergent bottles, milk jugs and molded plastic cases
- Low-density polyethylene (LDPE) – outdoor furniture, siding, floor tiles, shower curtains and clamshell packaging
- Polyethylene terephthalate (PET) – carbonated drinks bottles, peanut butter jars, plastic film and microwavable packaging
- Polypropylene (PP) – bottle caps, drinking straws, yogurt containers, appliances, car fenders (bumpers) and plastic pressure pipe systems
- Polystyrene (PS) – foam peanuts, food containers, plastic tableware, disposable cups, plates, cutlery, compact-disc (CD) and cassette boxes
- High impact polystyrene (HIPS) – refrigerator liners, food packaging and vending cups
- Polyurethanes (PU) – cushioning foams, thermal insulation foams, surface coatings and printing rollers: currently the sixth or seventh most commonly-used plastic, for instance the most commonly used plastic in cars
- Polyvinyl chloride (PVC) – plumbing pipes and guttering, electrical wire/cable insulation, shower curtains, window frames and flooring
- Polyvinylidene chloride (PVDC) – food packaging, such as: Saran
- Acrylonitrile butadiene styrene (ABS) – electronic equipment cases (e.g. computer monitors, printers, keyboards) and drainage pipe
- Polycarbonate+Acrylonitrile Butadiene Styrene (PC+ABS) – a blend of PC and ABS that creates a stronger plastic used in car interior and exterior parts, and mobile phone bodies
- Polyethylene+Acrylonitrile Butadiene Styrene (PE+ABS) – a slippery blend of PE and ABS used in low-duty dry bearings
Specialist plastics
- Polyepoxide (epoxy) – used as an adhesive, potting agent for electrical components, and matrix for composite materials with hardeners including amine, amide, and boron trifluoride
- Polymethyl methacrylate (PMMA) (acrylic) – contact lenses (of the original "hard" variety), glazing (best known in this form by its various trade names around the world; e.g. Perspex, Plexiglas, Oroglas), aglets, fluorescent light diffusers, rear light covers for vehicles. It forms the basis of artistic and commercial acrylic paints when suspended in water with the use of other agents.
- Polytetrafluoroethylene (PTFE), or Teflon – heat-resistant, low-friction coatings, used in things like non-stick surfaces for frying pans, plumber's tape and water slides
- Phenolics or phenol formaldehyde (PF) – high modulus, relatively heat resistant, and excellent fire resistant polymer. Used for insulating parts in electrical fixtures, paper laminated products (e.g. Formica), thermally insulation foams. It is a thermosetting plastic, with the familiar trade name Bakelite, that can be molded by heat and pressure when mixed with a filler-like wood flour or can be cast in its unfilled liquid form or cast as foam (e.g. Oasis). Problems include the probability of moldings naturally being dark colors (red, green, brown), and as thermoset it is difficult to recycle.
- Melamine formaldehyde (MF) – one of the aminoplasts, used as a multi-colorable alternative to phenolics, for instance in moldings (e.g. break-resistance alternatives to ceramic cups, plates and bowls for children) and the decorated top surface layer of the paper laminates (e.g. Formica)
- Urea-formaldehyde (UF) – one of the aminoplasts, used as a multi-colorable alternative to phenolics: used as a wood adhesive (for plywood, chipboard, hardboard) and electrical switch housings.
- Polyetheretherketone (PEEK) – strong, chemical- and heat-resistant thermoplastic, biocompatibility allows for use in medical implant applications, aerospace moldings. One of the most expensive commercial polymers.
- Maleimide/bismaleimide – used in high temperature composite materials
- Polyetherimide (PEI) (Ultem) – a high temperature, chemically stable polymer that does not crystallize
- Polyimide – a high temperature plastic used in materials such as Kapton tape
- Plastarch material – biodegradable and heat-resistant thermoplastic composed of modified corn starch
- Polylactic acid (PLA) – a biodegradable, thermoplastic found converted into a variety of aliphatic polyesters derived from lactic acid, which in turn can be made by fermentation of various agricultural products such as cornstarch, once made from dairy products
- Furan – resin based on furfuryl alcohol used in foundry sands and biologically derived composites
- Silicone poly (diketoenamine heat resistant resin used mainly as a sealant but also used for high temperature cooking utensils and as a base resin for industrial paints
- Polysulfone – high temperature melt processable resin used in membranes, filtration media, water heater dip tubes and other high temperature applications
- Polydiketoenamine (PDK) – a new type of plastic that can be dunked in acid and reshaped endlessly, currently being lab tested.[18]
History
_bowl%2C_by_GEECO%2C_Made_in_England%2C_c1950.jpg)
The development of plastics has evolved from the use of natural plastic materials (e.g., chewing gum, shellac) to the use of chemically modified, natural materials (e.g., natural rubber, nitrocellulose, collagen, galalite) and finally to completely synthetic molecules (e.g., bakelite, epoxy, polyvinyl chloride). Early plastics were bio-derived materials such as egg and blood proteins, which are organic polymers. In around 1600 BC, Mesoamericans used natural rubber for balls, bands, and figurines.[2] Treated cattle horns were used as windows for lanterns in the Middle Ages. Materials that mimicked the properties of horns were developed by treating milk-proteins (casein) with lye.
In the nineteenth century, as industrial chemistry developed during the Industrial Revolution, many materials were reported. The development of plastics also accelerated with Charles Goodyear's discovery of vulcanization to thermoset materials derived from natural rubber.

Parkesine (nitrocellulose) is considered the first man-made plastic. The plastic material was patented by Alexander Parkes, in Birmingham, England in 1856.[19] It was unveiled at the 1862 Great International Exhibition in London.[20] Parkesine won a bronze medal at the 1862 World's fair in London. Parkesine was made from cellulose (the major component of plant cell walls) treated with nitric acid as a solvent. The output of the process (commonly known as cellulose nitrate or pyroxilin) could be dissolved in alcohol and hardened into a transparent and elastic material that could be molded when heated.[21] By incorporating pigments into the product, it could be made to resemble ivory.
In 1897, the Hanover, Germany mass printing press owner Wilhelm Krische was commissioned to develop an alternative to blackboards.[22] The resultant horn-like plastic made from the milk protein casein was developed in cooperation with the Austrian chemist (Friedrich) Adolph Spitteler (1846–1940). The final result was unsuitable for the original purpose.[23] In 1893, French chemist Auguste Trillat discovered the means to insolubilize casein by immersion in formaldehyde, producing material marketed as galalith.[22]
In the early 1900s, Bakelite, the first fully synthetic thermoset, was reported by Belgian chemist Leo Baekeland by using phenol and formaldehyde.
After World War I, improvements in chemical technology led to an explosion in new forms of plastics, with mass production beginning in the 1940s and 1950s (around World War II).[24] Among the earliest examples in the wave of new polymers were polystyrene (PS), first produced by BASF in the 1930s,[2] and polyvinyl chloride (PVC), first created in 1872 but commercially produced in the late 1920s.[2] In 1923, Durite Plastics Inc. was the first manufacturer of phenol-furfural resins.[25] In 1933, polyethylene was discovered by Imperial Chemical Industries (ICI) researchers Reginald Gibson and Eric Fawcett.[2]
In 1954, polypropylene was discovered by Giulio Natta and began to be manufactured in 1957.[2]
In 1954, expanded polystyrene (used for building insulation, packaging, and cups) was invented by Dow Chemical.[2] The discovery of polyethylene terephthalate (PET) is credited to employees of the Calico Printers' Association in the UK in 1941; it was licensed to DuPont for the US and ICI otherwise, and as one of the few plastics appropriate as a replacement for glass in many circumstances, resulting in widespread use for bottles in Europe.[2]
Plastics industry
Plastics manufacturing is a major part of the chemical industry, and some of the world's largest chemical companies have been involved since the earliest days, such as the industry leaders BASF and Dow Chemical.
In 2014, sales of the top fifty companies amounted to US$961,300,000,000.[26] The firms came from some eighteen countries in total, with more than half of the companies on the list being headquartered in the US. Many of the top fifty plastics companies were concentrated in just three countries:
- United States: 12
- Japan: 8
- Germany: 6
BASF was the world's largest chemical producer for the ninth year in a row.[26]
Trade associations which represent the industry in the US include the American Chemistry Council.
Industry standards
Many of the properties of plastics are determined by standards specified by ISO, such as:
- ISO 306 – Thermoplastics
Many of the properties of plastics are determined by the UL Standards, tests specified by Underwriters Laboratories (UL), such as:
- Flammability – UL94
- High voltage arc tracking rate – UL746A
- Comparative Tracking Index
Additives
Blended into most plastics are additional organic or inorganic compounds. The average content of additives is a few percent. Many of the controversies associated with plastics actually relate to the additives:[27] organotin compounds are particularly toxic.[28]
Typical additives include:
Stabilizers
Polymer stabilizers prolong the lifetime of the polymer by suppressing degradation that results from UV-light, oxidation, and other phenomena. Typical stabilizers thus absorb UV light or function as antioxidants.
Fillers
Many plastics contain fillers, to improve performance or reduce production costs.[29] Typically fillers are mineral in origin, e.g., chalk. Other fillers include: starch, cellulose, wood flour, ivory dust and zinc oxide.
- most fillers are relatively inert and inexpensive materials, make the product cheaper by weight.
- stabilizing additives include fire retardants, to lower the flammability of the material.
- some fillers are more chemically active and are called: reinforcing agents.[30]
Plasticizers
Plasticizers are, by mass, often the most abundant additives.[28] These oily but nonvolatile compounds are blended in to plastics to improve rheology, as many organic polymers are otherwise too rigid for particular applications.
_phthalate.svg.png) Dioctyl phthalate is the most common plasticizer.
Dioctyl phthalate is the most common plasticizer.
Colorants
Colorants are another common additive, though their weight contribution is small.
Toxicity
Pure plastics have low toxicity due to their insolubility in water and because they are biochemically inert, due to a large molecular weight. Plastic products contain a variety of additives, some of which can be toxic.[31] For example, plasticizers like adipates and phthalates are often added to brittle plastics like polyvinyl chloride to make them pliable enough for use in food packaging, toys, and many other items. Traces of these compounds can leach out of the product. Owing to concerns over the effects of such leachates, the European Union has restricted the use of DEHP (di-2-ethylhexyl phthalate) and other phthalates in some applications, and the United States has limited the use of DEHP, DPB, BBP, DINP, DIDP, and DnOP in children's toys and child care articles with the Consumer Product Safety Improvement Act. Some compounds leaching from polystyrene food containers have been proposed to interfere with hormone functions and are suspected human carcinogens.[32] Other chemicals of potential concern include alkylphenols.[28]
Whereas the finished plastic may be non-toxic, the monomers used in the manufacture of the parent polymers may be toxic. In some cases, small amounts of those chemicals can remain trapped in the product unless suitable processing is employed. For example, the World Health Organization's International Agency for Research on Cancer (IARC) has recognized vinyl chloride, the precursor to PVC, as a human carcinogen.[32]
Bisphenol A (BPA)
Some polymers may also decompose into the monomers or other toxic substances when heated. In 2011, it was reported that "almost all plastic products" sampled released chemicals with estrogenic activity, although the researchers identified plastics which did not leach chemicals with estrogenic activity.[33]
The primary building block of polycarbonates, bisphenol A (BPA), is an estrogen-like endocrine disruptor that may leach into food.[32] Research in Environmental Health Perspectives finds that BPA leached from the lining of tin cans, dental sealants and polycarbonate bottles can increase body weight of lab animals' offspring.[34] A more recent animal study suggests that even low-level exposure to BPA results in insulin resistance, which can lead to inflammation and heart disease.[35]
As of January 2010, the LA Times newspaper reports that the United States FDA is spending $30 million to investigate indications of BPA being linked to cancer.[36]
Bis(2-ethylhexyl) adipate, present in plastic wrap based on PVC, is also of concern, as are the volatile organic compounds present in new car smell.
The European Union has a permanent ban on the use of phthalates in toys. In 2009, the United States government banned certain types of phthalates commonly used in plastic.[37]
Environmental effects

Most plastics are durable and degrade very slowly, as their chemical structure renders them resistant to many natural processes of degradation. There are differing estimates of how much plastic waste has been produced in the last century. By one estimate, one billion tons of plastic waste have been discarded since the 1950s.[38] Others estimate a cumulative human production of 8.3 billion tons of plastic of which 6.3 billion tons is waste, with a recycling rate of only 9%.[39] Much of this material may persist for centuries or longer, given the demonstrated persistence of structurally similar natural materials such as amber.
The Ocean Conservancy reported that China, Indonesia, Philippines, Thailand, and Vietnam dump more plastic in the sea than all other countries combined.[40] The rivers Yangtze, Indus, Yellow River, Hai River, Nile, Ganges, Pearl River, Amur, Niger, and the Mekong "transport 88–95% of the global [plastics] load into the sea."[41][42]
The presence of plastics, particularly microplastics, within the food chain is increasing. In the 1960s microplastics were observed in the guts of seabirds, and since then have been found in increasing concentrations.[43] The long-term effects of plastic in the food chain are poorly understood. In 2009, it was estimated that 10% of modern waste was plastic,[24] although estimates vary according to region.[43] Meanwhile, 50–80% of debris in marine areas is plastic.[43]
Prior to the Montreal Protocol, CFCs were commonly used in the manufacture of polystyrene, and as such the production of polystyrene contributed to the depletion of the ozone layer.
Climate change
In 2019, the Center for International Environmental Law published a new report on the impact of plastic on climate change. According to the report plastic will contribute Greenhouse gases in the equivalent of 850 million tons of Carbon dioxide (CO2) to the atmosphere in 2019. In current trend, annual emissions will grow to 1.34 billion tons by 2030. By 2050 plastic could emit 56 billion tons of Greenhouse gas emissions, as much as 14 percent of the earth's remaining carbon budget.[44]
The effect of plastics on global warming is mixed. Plastics are generally made from petroleum. If the plastic is incinerated, it increases carbon emissions; if it is placed in a landfill, it becomes a carbon sink[45] although biodegradable plastics have caused methane emissions. [46] Due to the lightness of plastic versus glass or metal, plastic may reduce energy consumption. For example, packaging beverages in PET plastic rather than glass or metal is estimated to save 52% in transportation energy.[2]
Production of plastics

Production of plastics from crude oil requires 62 to 108 MJ/Kg (taking into account the average efficiency of US utility stations of 35%). Producing silicon and semiconductors for modern electronic equipment is even more energy consuming: 230 to 235 MJ/Kg of silicon, and about 3,000 MJ/Kg of semiconductors.[47] This is much higher than the energy needed to produce many other materials, e.g. iron (from iron ore) requires 20-25 MJ/Kg of energy, glass (from sand, etc.) 18–35 MJ/Kg, steel (from iron) 20–50 MJ/Kg, paper (from timber) 25–50 MJ/Kg.[48]
Incineration of plastics
Controlled high-temperature incineration, above 850 °C for two seconds, performed with selective additional heating, breaks down toxic dioxins and furans from burning plastic, and is widely used in municipal solid waste incineration. Municipal solid waste incinerators also normally include flue gas treatments to reduce pollutants further. This is needed because uncontrolled incineration of plastic produces polychlorinated dibenzo-p-dioxins, a carcinogen (cancer causing chemical). The problem occurs because the heat content of the waste stream varies.[49] Open-air burning of plastic occurs at lower temperatures, and normally releases such toxic fumes.
Pyrolytic disposal
Plastics can be pyrolyzed into hydrocarbon fuels, since plastics include hydrogen and carbon. One kilogram of waste plastic produces roughly a liter of hydrocarbon.[50]
Decomposition of plastics
Plastics contribute to approximately 10% of discarded waste. Depending on their chemical composition, plastics and resins have varying properties related to contaminant absorption and adsorption. Polymer degradation takes much longer as a result of saline environments and the cooling effect of the sea. These factors contribute to the persistence of plastic debris in certain environments.[43] Recent studies have shown that plastics in the ocean decompose faster than was once thought, due to exposure to sun, rain, and other environmental conditions, resulting in the release of toxic chemicals such as bisphenol A. However, due to the increased volume of plastics in the ocean, decomposition has slowed down.[51] The Marine Conservancy has predicted the decomposition rates of several plastic products. It is estimated that a foam plastic cup will take 50 years, a plastic beverage holder will take 400 years, a disposable nappy will take 450 years, and fishing line will take 600 years to degrade.[52]
In 2018, a survey by the Global Oceanic Environmental Survey (GOES) Foundation found that the ecosystem in seas and oceans may collapse in the next 25 years, potentially causing failure of terrestrial ecosystem and "very possibly the end of life on Earth as we know it";[53] the main agents of this prediction were hypothesized to be plastic, ocean acidification, and ocean pollution. In order to prevent such a catastrophe, experts have proposed a total single-use plastic ban, wood burning bans while planting "as many trees as possible," "pollution-free recycling of electronics, and by 2030 all industries to be zero toxic discharge." One British scientist advocates "special protection and perservation of peat bogs, wetlands, marshlands and mangrove swamps to ensure carbon dioxide is absorbed from the atmosphere."[53]
Microbial species capable of degrading plastics are known to science, and some are potentially useful for the disposal of certain classes of plastic waste.
- In 1975 a team of Japanese scientists studying ponds containing waste water from a nylon factory, discovered a strain of Flavobacterium that digested certain byproducts of nylon 6 manufacture, such as the linear dimer of 6-aminohexanoate.[54] Nylon 4 or polybutyrolactam can be degraded by the (ND-10 and ND-11) strands of Pseudomonas sp. found in sludge. This produced γ-aminobutyric acid (GABA) as a byproduct.[55]
- Several species of soil fungi can consume polyurethane.[56] This includes two species of the Ecuadorian fungus Pestalotiopsis that can consume polyurethane aerobically and also in anaerobic conditions such as those at the bottom of landfills.[57]
- Methanogenic consortia degrade styrene, using it as a carbon source.[58] Pseudomonas putida can convert styrene oil into various biodegradable polyhydroxyalkanoates.[59][60]
- Microbial communities isolated from soil samples mixed with starch have been shown to be capable of degrading polypropylene.[61]
- The fungus Aspergillus fumigatus effectively degrades plasticized PVC.[62] Phanerochaete chrysosporium has been grown on PVC in a mineral salt agar.[63] Phanerochaete chrysosporium, Lentinus tigrinus, Aspergillus niger, and Aspergillus sydowii can also effectively degrade PVC.[64] Phanerochaete chrysosporium was grown on PVC in a mineral salt agar.[63]
- Acinetobacter has been found to partially degrade low molecular weight polyethylene oligomers.[55] When used in combination, Pseudomonas fluorescens and Sphingomonas can degrade over 40% of the weight of plastic bags in less than three months.[65] The thermophilic bacterium Brevibacillus borstelensis (strain 707) was isolated from a soil sample and found capable of using low-density polyethylene as a sole carbon source when incubated at 50 degrees Celsius. Pre-exposure of the plastic to ultraviolet radiation broke chemical bonds and aided biodegradation; the longer the period of UV exposure, the greater the promotion of the degradation.[66]
- Less desirably, hazardous molds have been found aboard space stations, molds that degrade rubber into a digestible form.[67]
- Several species of yeasts, bacteria, algae and lichens have been found growing on synthetic polymer artifacts in museums and at archaeological sites.[68]
- In the plastic-polluted waters of the Sargasso Sea, bacteria have been found that consume various types of plastic; however it is unknown to what extent these bacteria effectively clean up poisons rather than simply releasing them into the marine microbial ecosystem.
- Plastic eating microbes also have been found in landfills.[69]
- Nocardia can degrade PET with an esterase enzyme.
- The fungus Geotrichum candidum, found in Belize, has been found to consume the polycarbonate plastic found in CDs.[70][71]
- Phenol-formaldehyde, commonly known as bakelite, is degraded by the white rot fungus Phanerochaete chrysosporium.[72]
- The futuro house was made of fibreglass-reinforced polyesters, polyester-polyurethane, and poly(methylmethacrylate). One such house was found to be harmfully degraded by Cyanobacteria and Archaea.[73][74]
Recycling
Thermoplastics can be remelted and reused, and thermoset plastics can be ground up and used as filler, although the purity of the material tends to degrade with each reuse cycle. There are methods by which plastics can be broken down to a feedstock state.
The greatest challenge to the recycling of plastics is the difficulty of automating the sorting of plastic wastes, making it labor-intensive. Typically, workers sort the plastic by looking at the resin identification code, although common containers like soda bottles can be sorted from memory. Typically, the caps for PETE bottles are made from a different kind of plastic which is not recyclable, which presents additional problems for the sorting process. Other recyclable materials such as metals are easier to process mechanically. However, new processes of mechanical sorting are being developed to increase the capacity and efficiency of plastic recycling.
While containers are usually made from a single type and color of plastic, making them relatively easy to sort, a consumer product like a cellular phone may have many small parts consisting of over a dozen different types and colors of plastics. In such cases, the resources it would take to separate the plastics far exceed their value and the item is discarded. However, developments are taking place in the field of active disassembly, which may result in more product components being reused or recycled. Recycling certain types of plastics can be unprofitable as well. For example, polystyrene is rarely recycled because the process is usually not cost effective. These unrecycled wastes are typically disposed of in landfills, incinerated or used to produce electricity at waste-to-energy plants.
An early success in the recycling of plastics is Vinyloop, an industrial process to separate PVC from other materials through dissolution, filtration and separation of contaminants. A solvent is used in a closed loop to elute PVC from the waste. This makes it possible to recycle composite PVC waste, which is normally incinerated or put in a landfill. Vinyloop-based recycled PVC's primary energy demand is 46 percent lower than conventionally produced PVC. The global warming potential is 39 percent lower. This is why the use of recycled material leads to a significantly better ecological outcome.[75] This process was used after the Olympic Games in London 2012. Parts of temporary Buildings like the Water Polo Arena and the Royal Artillery Barracks were recycled. In this way, the PVC Policy could be fulfilled, which says that no PVC waste should be left after the games had ended.[76]
In 1988, to assist recycling of disposable items, the Plastic Bottle Institute of the U.S. Society of the Plastics Industry devised a now-familiar scheme to mark plastic bottles by plastic type. Under this scheme, a plastic container is marked with a triangle which encloses a number denoting the plastic type. This scheme was added to the ASTM Standard ASTM D7611[77]:







- Polyethylene terephthalate (PET or PETE)
- High-density polyethylene (HDPE)
- Polyvinyl chloride (PVC)
- Low-density polyethylene (LDPE)
- Polypropylene (PP)
- Polystyrene (PS)
- Other types of plastic (see list below)
Representative polymers


Bakelite
The first plastic based on a synthetic polymer was made from phenol and formaldehyde, with the first viable and cheap synthesis methods invented in 1907, by Leo Hendrik Baekeland, a Belgian-born American living in New York state. Baekeland was looking for an insulating shellac to coat wires in electric motors and generators. He found that combining phenol (C6H5OH) and formaldehyde (HCOH) formed a sticky mass and later found that the material could be mixed with wood flour, asbestos, or slate dust to create strong and fire resistant "composite" materials. The new material tended to foam during synthesis, requiring that Baekeland build pressure vessels to force out the bubbles and provide a smooth, uniform product, as he announced in 1909, in a meeting of the American Chemical Society.[79] Bakelite was originally used for electrical and mechanical parts, coming into widespread use in consumer goods and jewelry in the 1920s. Bakelite was a purely synthetic material, not derived from living matter. It was also an early thermosetting plastic.
Polystyrene
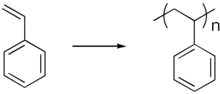
Unplasticised polystyrene is a rigid, brittle, inexpensive plastic that has been used to make plastic model kits and similar knick-knacks. It also is the basis for some of the most popular "foamed" plastics, under the name styrene foam or Styrofoam. Like most other foam plastics, foamed polystyrene can be manufactured in an "open cell" form, in which the foam bubbles are interconnected, as in an absorbent sponge, and "closed cell", in which all the bubbles are distinct, like tiny balloons, as in gas-filled foam insulation and flotation devices. In the late 1950s, high impact styrene was introduced, which was not brittle. It finds much current use as the substance of toy figurines and novelties.
Polyvinyl chloride

Polyvinyl chloride (PVC, commonly called "vinyl")[80] incorporates chlorine atoms. The C-Cl bonds in the backbone are hydrophobic and resist oxidation (and burning). PVC is stiff, strong, heat and weather resistant, properties that recommend its use in devices for plumbing, gutters, house siding, enclosures for computers and other electronics gear. PVC can also be softened with chemical processing, and in this form it is now used for shrink-wrap, food packaging, and rain gear.
All PVC polymers are degraded by heat and light. When this happens, hydrogen chloride is released into the atmosphere and oxidation of the compound occurs.[81] Because hydrogen chloride readily combines with water vapor in the air to form hydrochloric acid,[82] polyvinyl chloride is not recommended for long-term archival storage of silver, photographic film or paper (mylar is preferable).[83]
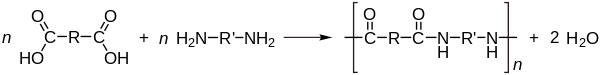
Nylon
The plastics industry was revolutionized in the 1930s with the announcement of polyamide (PA), far better known by its trade name nylon. Nylon was the first purely synthetic fiber, introduced by DuPont Corporation at the 1939 World's Fair in New York City.
In 1927, DuPont had begun a secret development project designated Fiber66, under the direction of Harvard chemist Wallace Carothers and chemistry department director Elmer Keiser Bolton. Carothers had been hired to perform pure research, and he worked to understand the new materials' molecular structure and physical properties. He took some of the first steps in the molecular design of the materials.
His work led to the discovery of synthetic nylon fiber, which was very strong but also very flexible. The first application was for bristles for toothbrushes. However, Du Pont's real target was silk, particularly silk stockings. Carothers and his team synthesized a number of different polyamides including polyamide 6.6 and 4.6, as well as polyesters.[84]
It took DuPont twelve years and US$27 million to refine nylon, and to synthesize and develop the industrial processes for bulk manufacture. With such a major investment, it was no surprise that Du Pont spared little expense to promote nylon after its introduction, creating a public sensation, or "nylon mania".
Nylon mania came to an abrupt stop at the end of 1941 when the US entered World War II. The production capacity that had been built up to produce nylon stockings, or just nylons, for American women was taken over to manufacture vast numbers of parachutes for fliers and paratroopers. After the war ended, DuPont went back to selling nylon to the public, engaging in another promotional campaign in 1946 that resulted in an even bigger craze, triggering the so-called nylon riots.
Subsequently, polyamides 6, 10, 11, and 12 have been developed based on monomers which are ring compounds; e.g. caprolactam. Nylon 66 is a material manufactured by condensation polymerization.
Nylons still remain important plastics, and not just for use in fabrics. In its bulk form it is very wear resistant, particularly if oil-impregnated, and so is used to build gears, plain bearings, valve seats, seals and because of good heat-resistance, increasingly for under-the-hood applications in cars, and other mechanical parts.
Poly(methyl methacrylate)
Poly(methyl methacrylate) (PMMA), also known as acrylic or acrylic glass as well as by the trade names Plexiglas, Acrylite, Lucite, and Perspex among several others (see below), is a transparent thermoplastic often used in sheet form as a lightweight or shatter-resistant alternative to glass. The same material can be utilised as a casting resin, in inks and coatings, and has many other uses.
Rubber
Natural rubber is an elastomer (an elastic hydrocarbon polymer) that originally was derived from latex, a milky colloidal suspension found in specialised vessels in some plants. It is useful directly in this form (indeed, the first appearance of rubber in Europe was cloth waterproofed with unvulcanized latex from Brazil). However, in 1839, Charles Goodyear invented vulcanized rubber; a form of natural rubber heated with sulfur (and a few other chemicals), forming cross-links between polymer chains (vulcanization), improving elasticity and durability.
In 1851, Nelson Goodyear added fillers to natural rubber materials to form ebonite.[30]
Synthetic rubber
The first fully synthetic rubber was synthesized by Sergei Lebedev in 1910. In World War II, supply blockades of natural rubber from South East Asia caused a boom in development of synthetic rubber, notably styrene-butadiene rubber. In 1941, annual production of synthetic rubber in the U.S. was only 231 tonnes which increased to 840,000 tonnes in 1945. In the space race and nuclear arms race, Caltech researchers experimented with using synthetic rubbers for solid fuel for rockets. Ultimately, all large military rockets and missiles would use synthetic rubber based solid fuels, and they would also play a significant part in the civilian space effort.
See also
- Corn construction
- Films
- Light activated resin
- Nurdle
- Molding (process)
- Injection molding
- Rotational molding
- Organic light emitting diode
- Plastic film
- Plastic recycling
- Plastics engineering
- Plastics extrusion
- Plasticulture
- Biodegradable plastic
- Bioplastic
- Organisms breaking down plastic
- Progressive bag alliance
- Roll-to-roll processing
- Self-healing plastic
- Thermal cleaning
- Thermoforming
- Timeline of materials technology
References
- Life cycle of a plastic product. Americanchemistry.com. Retrieved 2011-07-01.
- Andrady AL, Neal MA (July 2009). "Applications and societal benefits of plastics". Philos. Trans. R. Soc. Lond. B Biol. Sci. 364 (1526): 1977–84. doi:10.1098/rstb.2008.0304. PMC 2873019. PMID 19528050.
- American Chemical Society National Historic Chemical Landmarks. "Bakelite: The World's First Synthetic Plastic". Retrieved 23 February 2015.
- Edgar, David; Edgar, Robin (2009). Fantastic Recycled Plastic: 30 Clever Creations to Spark Your Imagination. Sterling Publishing Company, Inc. ISBN 978-1-60059-342-0 – via Google Books.
- Teegarden, David M. (2004). Polymer Chemistry: Introduction to an Indispensable Science. NSTA Press. ISBN 978-0-87355-221-9 – via Google Books.
- Plastikos, Henry George Liddell, Robert Scott, A Greek-English Lexicon, at Perseus. Perseus.tufts.edu. Retrieved on 2011-07-01.
- Plastic, Online Etymology Dictionary. Etymonline.com. Retrieved on 2011-07-01.
- Ebbing, Darrell; Gammon, Steven D. (2016). General Chemistry. Cengage Learning. ISBN 978-1-305-88729-9.
- Classification of Plastics Archived 2007-12-15 at the Wayback Machine. Dwb.unl.edu. Retrieved on 2011-07-01.
- Periodic Table of Polymers Archived 2008-07-03 at the Wayback Machine Dr Robin Kent – Tangram Technology Ltd.
- Composition and Types of Plastic Inforplease website
- Gilleo, Ken (2004). Area Array Packaging Processes: For BGA, Flip Chip, and CSP. McGraw Hill Professional. ISBN 978-0-07-142829-3.
- Kutz, Myer (2002). Handbook of Materials Selection. John Wiley & Sons. ISBN 978-0-471-35924-1.
- Heeger, A.J.; Schrieffer, J.R.; Su, W.-P.; Su, W. (1988). "Solitons in conducting polymers". Reviews of Modern Physics. 60 (3): 781–850. Bibcode:1988RvMP...60..781H. doi:10.1103/RevModPhys.60.781.
- Brandl, Helmut; Püchner, Petra (1992). "Biodegradation Biodegradation of plastic bottles made from 'Biopol' in an aquatic ecosystem under in situ conditions". Biodegradation. 2 (4): 237–43. doi:10.1007/BF00114555.
- "Archived copy". Archived from the original on 2011-07-20. Retrieved 2011-03-24.CS1 maint: archived copy as title (link)
- Galie, Fabrizio (November 2016). "Global Market Trends and Investments in Polyethylene and Polyproplyene" (PDF). ICIS Whitepaper. Reed business Information, Inc. Retrieved 16 December 2017.
- "Scientists could have finally created the 'holy grail' of plastic". The Independent. 2019-05-09. Retrieved 2019-05-10.
- UK Patent office (1857). Patents for inventions. UK Patent office. p. 255.
- Fenichell, Stephen (1996). Plastic : the making of a synthetic century. New York: HarperBusiness. p. 17. ISBN 978-0-88730-732-4.
- "Dictionary – Definition of celluloid". Websters-online-dictionary.org. Archived from the original on 2009-12-11. Retrieved 2011-10-26.
- Christel Trimborn (August 2004). "Jewelry Stone Make of Milk". GZ Art+Design. Retrieved 2010-05-17.
- Trimborn, Christel (August 2004). "Jewelry Stone Make of Milk". GZ Art+Design. Missing or empty
|url=(help) - Thompson RC, Swan SH, Moore CJ, vom Saal FS (July 2009). "Our plastic age". Philos. Trans. R. Soc. Lond. B Biol. Sci. 364 (1526): 1973–76. doi:10.1098/rstb.2009.0054. PMC 2874019. PMID 19528049.
- "Historical Overview and Industrial Development". International Furan Chemicals, Inc. Retrieved 4 May 2014.
- Tullo, Alexander H. (27 July 2015). "Global Top 50 Chemical Companies". Chemical & Engineering News. American Chemical Society. Retrieved 27 October 2015.
- Hans-Georg Elias "Plastics, General Survey" in Ullmann's Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim. doi:10.1002/14356007.a20_543
- Teuten EL, Saquing JM, Knappe DR, et al. (July 2009). "Transport and release of chemicals from plastics to the environment and to wildlife". Philos. Trans. R. Soc. Lond. B Biol. Sci. 364 (1526): 2027–45. doi:10.1098/rstb.2008.0284. PMC 2873017. PMID 19528054.
- Kulshreshtha, A. K.; Vasile, Cornelia (2002). Handbook of Polymer Blends and Composites. iSmithers Rapra Publishing. ISBN 978-1-85957-249-8.
- Seymour, Raymond Benedict; Deaning, Rudolph D. (1987). History of Polymeric Composites. VSP. p. 374.
- Hahladakis, John N.; Velis, Costas A.; Weber, Roland; Iacovidou, Eleni; Purnell, Phil (February 2018). "An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling". Journal of Hazardous Materials. 344: 179–199. doi:10.1016/j.jhazmat.2017.10.014. PMID 29035713.

- McRandle, P.W. (March–April 2004). "Plastic Water Bottles". National Geographic. Retrieved 2007-11-13.
- Yang, Chun Z.; Yaniger, Stuart I.; Jordan, V. Craig; Klein, Daniel J.; Bittner, George D. (2 March 2011). "Most Plastic Products Release Estrogenic Chemicals: A Potential Health Problem That Can Be Solved". Environmental Health Perspectives. 119 (7): 989–96. doi:10.1289/ehp.1003220. PMC 3222987. PMID 21367689.
- Rubin, BS; Murray, MK; Damassa, DA; King, JC; Soto, AM (July 2001). "Perinatal exposure to low doses of bisphenol A affects body weight, patterns of estrous cyclicity, and plasma LH levels". Environmental Health Perspectives. 109 (7): 675–80. doi:10.2307/3454783. JSTOR 3454783. PMC 1240370. PMID 11485865.
- Alonso-Magdalena, Paloma; Morimoto, Sumiko; Ripoll, Cristina; Fuentes, Esther; Nadal, Angel (January 2006). "The Estrogenic Effect of Bisphenol A Disrupts Pancreatic β-Cell Function In Vivo and Induces Insulin Resistance". Environmental Health Perspectives. 114 (1): 106–12. doi:10.1289/ehp.8451. PMC 1332664. PMID 16393666. Archived from the original on 2009-01-19.
- Andrew Zajac FDA issues BPA guidelines, Los Angeles Times, January 16, 2010
- Lisa Wade McCormick More Kids' Products Found Containing Unsafe Chemicals, ConsumerAffairs.com, October 30, 2009
- Weisman, Alan (2007). The world without us. New York: Thomas Dunne Books/St. Martin's Press. ISBN 978-1-4434-0008-4.
- Geyer, Roland; et al. (19 July 2017). "Production, use, and fate of all plastics ever made". Science Advances. 3 (7): e1700782. Bibcode:2017SciA....3E0782G. doi:10.1126/sciadv.1700782. PMC 5517107. PMID 28776036.
- Hannah Leung (21 April 2018). "Five Asian Countries Dump More Plastic Into Oceans Than Anyone Else Combined: How You Can Help". Forbes. Retrieved 23 June 2019.
China, Indonesia, Philippines, Thailand, and Vietnam are dumping more plastic into oceans than the rest of the world combined, according to a 2017 report by Ocean Conservancy
- Christian Schmidt; Tobias Krauth; Stephan Wagner (11 October 2017). "Export of Plastic Debris by Rivers into the Sea". Environmental Science & Technology. 51 (21): 12246–12253. Bibcode:2017EnST...5112246S. doi:10.1021/acs.est.7b02368. PMID 29019247.
The 10 top-ranked rivers transport 88–95% of the global load into the sea
- Harald Franzen (30 November 2017). "Almost all plastic in the ocean comes from just 10 rivers". Deutsche Welle. Retrieved 18 December 2018.
It turns out that about 90 percent of all the plastic that reaches the world's oceans gets flushed through just 10 rivers: The Yangtze, the Indus, Yellow River, Hai River, the Nile, the Ganges, Pearl River, Amur River, the Niger, and the Mekong (in that order).
- Barnes DK, Galgani F, Thompson RC, Barlaz M (July 2009). "Accumulation and fragmentation of plastic debris in global environments". Philos. Trans. R. Soc. Lond. B Biol. Sci. 364 (1526): 1985–98. doi:10.1098/rstb.2008.0205. PMC 2873009. PMID 19528051.
- "Sweeping New Report on Global Environmental Impact of Plastics Reveals Severe Damage to Climate". Center for International Environmental Law (CIEL). Retrieved 16 May 2019.
- EPA. (2012). Landfilling.
- Levis, James W.; Barlaz, Morton A. (July 2011). "Is Biodegradability a Desirable Attribute for Discarded Solid Waste? Perspectives from a National Landfill Greenhouse Gas Inventory Model". Environmental Science & Technology. 45 (13): 5470–76. Bibcode:2011EnST...45.5470L. doi:10.1021/es200721s. PMID 21615182.
- "The monster footprint of digital technology". Low-Tech Magazine. Retrieved 2017-04-18.
- "How much energy does it take (on average) to produce 1 kilogram of the following materials?". Low-Tech Magazine. 2014-12-26. Retrieved 2017-04-18.
- Halden, RU (2010). "Plastics and Health Risks". Annual Review of Public Health. 31: 179–94. doi:10.1146/annurev.publhealth.012809.103714. PMID 20070188.
- The Hindu Dec. 12, 2005. Retrieved on 2011-07-01.
- Chemical Society, American. "Plastics In Oceans Decompose, Release Hazardous Chemicals, Surprising New Study Says". Science Daily. Science Daily. Retrieved 15 March 2015.
- Le Guern, Claire (March 2018). "When The Mermaids Cry: The Great Plastic Tide". Coastal Care. Archived from the original on 5 April 2018. Retrieved 10 November 2018.
- MURRAY, PAULA (2018-12-23). "'We've 10 years to save the seas or life on earth will become impossible'". Express. Retrieved 3 January 2019.
- Kinoshita, S.; Kageyama, S., Iba, K., Yamada, Y. and Okada, H. (1975). "Utilization of a cyclic dimer and linear oligomers of e-aminocaproic acid by Achromobacter guttatus". Agricultural and Biological Chemistry. 39 (6): 1219–23. doi:10.1271/bbb1961.39.1219. ISSN 0002-1369.CS1 maint: multiple names: authors list (link)
- Yutaka Tokiwa; Buenaventurada P. Calabia; Seiichi Aiba (September 2009). "Biodegradability of Plastics". International Journal of Molecular Sciences. 10 (9): 3722–44. doi:10.3390/ijms10093722. PMC 2769161. PMID 19865515.
- Jonathan R. Russell; Jeffrey Huang; Scott A. Strobel (September 2011). "Biodegradation of Polyester Polyurethane by Endophytic Fungi". Applied and Environmental Microbiology. 77 (17): 6076–84. doi:10.1128/aem.00521-11. PMC 3165411. PMID 21764951.
- Russell, Jonathan R.; Huang, Jeffrey; Anand, Pria; Kucera, Kaury; Sandoval, Amanda G.; Dantzler, Kathleen W.; Hickman, Dashawn; Jee, Justin; Kimovec, Farrah M.; Koppstein, David; Marks, Daniel H.; Mittermiller, Paul A.; Núñez, Salvador Joel; Santiago, Marina; Townes, Maria A.; Vishnevetsky, Michael; Williams, Neely E.; Vargas, Mario Percy Núñez; Boulanger, Lori-Ann; Bascom-Slack, Carol; Strobel, Scott A. (July 2011). "Biodegradation of Polyester Polyurethane by Endophytic Fungi". Applied and Environmental Microbiology. 77 (17): 6076–84. doi:10.1128/AEM.00521-11. PMC 3165411. PMID 21764951.
- "Deep Geologic Repository Project" (PDF). Ceaa-acee.gc.ca. Retrieved 2017-04-18.
- Roy, Robert (2006-03-07). "Immortal Polystyrene Foam Meets its Enemy". Livescience.com. Retrieved 2017-04-18.
- Ward, PG; Goff, M; Donner, M; Kaminsky, W; O'Connor, KE. (2006). "A two step chemo-biotechnological conversion of polystyrene to a biodegradable thermoplastic". Environmental Science and Technology. 40 (7): 2433–37. Bibcode:2006EnST...40.2433W. doi:10.1021/es0517668. PMID 16649270.
- Cacciari I; Quatrini P; Zirletta G; Mincione E; Vinciguerra V; Lupattelli P; Giovannozzi Sermanni G (1993). "Isotactic polypropylene biodegradation by a microbial community: physicochemical characterization of metabolites produced". Applied and Environmental Microbiology. 59 (11): 3695–3700. doi:10.1128/AEM.59.11.3695-3700.1993.
- Ishtiaq Ali, Muhammad (2011). Microbial degradation of polyvinyl chloride plastics (PDF) (Ph.D.). Quaid-i-Azam University. pp. 45–46.
- Ishtiaq Ali, Muhammad (2011). Microbial degradation of polyvinyl chloride plastics (PDF) (Ph.D.). Quaid-i-Azam University. p. 76.
- Ishtiaq Ali, Muhammad (2011). Microbial degradation of polyvinyl chloride plastics (PDF) (Ph.D.). Quaid-i-Azam University. p. 122.
- "CanadaWorld – WCI student isolates microbe that lunches on plastic bags". The Record.com. Archived from the original on 2011-07-18.
- Hadad D; Geresh S; Sivan A (2005). "Biodegradation of polyethylene by the thermophilic bacterium Brevibacillus borstelensis". Journal of Applied Microbiology. 98 (5): 1093–100. doi:10.1111/j.1365-2672.2005.02553.x. PMID 15836478.
- Trudy E. Bell (2007). "Preventing "Sick" Spaceships".
- Francesca Cappitelli; Claudia Sorlini (2008). "Microorganisms Attack Synthetic Polymers in Items Representing Our Cultural Heritage". Applied and Environmental Microbiology. 74 (3): 564–69. doi:10.1128/AEM.01768-07. PMC 2227722. PMID 18065627.
- Gwyneth Dickey Zaikab (March 2011). "Marine microbes digest plastic". Nature. doi:10.1038/news.2011.191.
- Bosch, Xavier (2001). "Fungus eats CD". Nature. doi:10.1038/news010628-11.
- "Fungus 'eats' CDs". BBC. June 2001.
- Gusse AC; Miller PD; Volk TJ (July 2006). "White-rot fungi demonstrate first biodegradation of phenolic resin". Environmental Science and Technology. 40 (13): 4196–99. Bibcode:2006EnST...40.4196G. doi:10.1021/es060408h. PMID 16856735.
- Cappitelli F; Principi P; Sorlini C. (Aug 2006). "Biodeterioration of modern materials in contemporary collections: can biotechnology help?". Trends in Biotechnology. 24 (8): 350–54. doi:10.1016/j.tibtech.2006.06.001. PMID 16782219.
- Andrea Rinaldi (November 7, 2006). "Saving a fragile legacy. Biotechnology and microbiology are increasingly used to preserve and restore the worlds cultural heritage". EMBO Reports. 7 (11): 1075–79. doi:10.1038/sj.embor.7400844. PMC 1679785. PMID 17077862.
- "Solvay, asking more from chemistry" (PDF). Archived from the original (PDF) on 2016-05-16.
- "Implementation of the PVC policy". The London Organising Committee of the Olympic Games and Paralympic Games Limited. Retrieved October 24, 2012.
- "ASTM D7611 - STANDARD PRACTICE FOR CODING PLASTIC MANUFACTURED ARTICLES FOR RESIN IDENTIFICATION" (PDF). ASTM International.
- SPI Resin Identification Code – Guide to Correct Use Archived 2012-03-21 at the Wayback Machine. plasticsindustry.org
- Watson, Peter. A Terrible Beauty (also published as Modern Mind: An intellectual history of the 20th century). London: Weidenfeld & Nicolson Ltd (imprint of Orion Books). 2001
- Jezek, Geno. "What is Vinyl?". Retrieved 9 January 2011.
- "Polyvinyl chloride". Plasticsusa.com. Archived from the original on 15 July 2011. Retrieved 9 January 2011.
- Salocks, Charles & Kaley, Karlyn Black (2 February 2004). "Technical Support Document: Toxicology of Hydrogen Chloride (Revised)" (PDF). California EPA, Office of Environmental Health Hazard Assessment. p. 8. Archived from the original (PDF) on 4 November 2010. Retrieved 9 January 2011.
- "How can I preserve my family photographs for my grandchildren?". The Library of Congress Preservation FAQs. LoC. Retrieved 9 January 2011.
- Kinnane, Adrian (2002). DuPont: From the banks of the Brandywine to miracles of science. Baltimore, MD: Johns Hopkins University Press. pp. 116–125. ISBN 978-0-8018-7059-0.
- Substantial parts of this text originated from An Introduction to Plastics v1.0 by Greg Goebel (1 March 2001), which is in the public domain.
External links
| Wikimedia Commons has media related to Plastics. |
| Wikiquote has quotations related to: Plastic |
- J. Harry Dubois Collection on the History of Plastics, ca. 1900–1975 Archives Center, National Museum of American History, Smithsonian Institution.
- Material Properties of Plastics – Mechanical, Thermal & Electrical Properties
- List of over 600 plastics
- Plastics Historical Society
- History of plastics, Society of the Plastics Industry
- "A brief history of plastics, natural and synthetic", from BBC Magazine
- Timeline of important milestone of plastic injection moulding and plastics