Polyacrylic acid
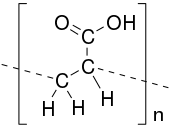
Poly(acrylic acid) (PAA; trade name Carbomer) is a synthetic high-molecular weight polymer of acrylic acid. The IUPAC name is poly(1-carboxyethylene). They may be homopolymers of acrylic acid, or crosslinked with an allyl ether of pentaerythritol, allyl ether of sucrose, or allyl ether of propylene. In a water solution at neutral pH, PAA is an anionic polymer, i.e. many of the side chains of PAA will lose their protons and acquire a negative charge. This makes PAAs polyelectrolytes, with the ability to absorb and retain water and swell to many times their original volume.
 | |
| Names | |
|---|---|
| IUPAC name
Poly(acrylic acid) | |
| Other names
PAA, PAAc, Acrysol, Acumer, Alcosperse, Aquatreat, Carbomer, Sokalan | |
| Identifiers | |
| ChemSpider |
|
| ECHA InfoCard | 100.115.375 |
| KEGG | |
| UNII |
|
CompTox Dashboard (EPA) |
|
| Properties | |
| (C3H4O2)n | |
| Molar mass | variable |
| log P | 0.25700[1] |
| Hazards | |
EU classification (DSD) (outdated) |
|
| R-phrases (outdated) | R36 R37 R38 |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Dry PAAs are sold as white, fluffy powders that are frequently used as gels in cosmetic and personal care products. Their role in cosmetics is to suspend solid in liquids, prevent emulsions from separating and control the consistency in flow of cosmetics. Carbomer codes (910, 934, 940, 941, and 934P) are an indication of molecular weight and the specific components of the polymer. For many applications PAAs are used in form of alkali metal or ammonium salts, e.g. sodium polyacrylate. In the dry powder form, the positively charged sodium ions are bound to the polyacrylate, however in aqueous solutions the sodium ions can dissociate. Instead of an organized polymer chain, this leads to a swollen gel that can absorb a high amount of water.
Polyacrylic acid is a weak anionic polyelectrolyte, whose degree of ionisation is dependent on solution pH. In its non-ionised form at low pHs, PAA may associate with various non-ionic polymers (such as polyethylene oxide, poly-N-vinyl pyrrolidone, polyacrylamide, and some cellulose ethers) and form hydrogen-bonded interpolymer complexes[2] In aqueous solutions PAA can also form polycomplexes with oppositely charged polymers (for example, chitosan), surfactants, and drug molecules (for example, streptomycin).[3]
Applications
Polyacrylic acid and its derivatives are used in disposable diapers,[4] ion exchange resins, adhesives and detergents. Detergents are often copolymers of acrylic acid that can be used in both zeolites and phosphates in washing powder formulations. They are also popular as thickening, dispersing, suspending, and emulsifying agents in pharmaceuticals, cosmetics, and paints. Cross-linked polyacrylic acid has also been used in the processing of household products, including floor cleaners. Acrylic acid is also the main component of Superadsorbent Polymers (SAPs), cross-linked polyacrylates that can absorb and retain more than 100 times of their own weight in liquid. [5] PAA may inactivate the antiseptic chlorhexidine gluconate.[6] The neutralized polyacrylic acid gels are suitable to obtain biocompatible matrices for medical applications such as gels for skin care or skin disease treatment products. PAA films can be deposited on orthopaedic implants to protect them from corrosion. Crosslinked hydrogels of AA and gelatin have also been used as medical glue, which has a high bonding strength. For the development of polymeric matrices which allows controlled delivery rate of active substances, the recent investigations aimed towards the clarification of the conformational changes of the polymeric gel during neutralization, light irradiation, and embedment of gold nanoparticles.[7][8] The US Food and Drug Administration authorised the use of SAPs in packaging with indirect food contact.
Polymer synthesis
Free radical polymerization is still the most common industrial for production of polymers. For the free radical polymerization of acrylic acid, most commonly thermochemical initiators such as potassium persulfate and AIBN are used. The polymerization can take several hours to complete but can be accelerated by drastically increasing the temperature and pressure. PAA is widely used in dispersants and since the molecular weight has a significant impact on the rheological properties and dispersion capacity, it is crucial to have control over the molecular weight distribution. RAFT polymerization was used to obtain PAA with a defined molecular weight, leading to improved dispersion properties. This was only possible in low AA/CTA ratios, at higher ratios transfer to the solvent was observed that had a negative effect on control over the reaction. Block copolymers of PAA have been prepared that are responsive to pH and temperature stimuli, demonstrating the possibility to be used for drug delivery purposes. ATRP has been used to growth PAA brushes from the surface. One reported approach was with surface-initiated ATRP of tert-butyl acrylate that was subsequently deprotected through pyrolysis to form the carboxylic acid. These PAA brushes can form chelate complex with silver and can therefore exhibit antibacterial activity.
See also
References
- "Polyacrylic acid_msds".
- Vitaliy V Khutoryanskiy, Georgios Staikos (2009) https://books.google.com/books?id=6vOLVWUSebYC&pg=PR5
- Nurkeeva, Zauresh S; Khutoryanskiy, Vitaliy V; Mun, Grigoriy A; Sherbakova, Marina V; Ivaschenko, Anatoliy T; Aitkhozhina, Nazira A (2004). "Polycomplexes of poly(acrylic acid) with streptomycin sulfate and their antibacterial activity". European Journal of Pharmaceutics and Biopharmaceutics. 57 (2): 245–9. doi:10.1016/S0939-6411(03)00149-8. PMID 15018981.
- "Acrylates". The Macrogalleria. Polymer Science Learning Center. 2005. Retrieved 2015-06-25.
- Orwoll, Robert A.; Yong, Chong S. (1999). "Poly(acrylic acid)". In Mark, James E. (ed.). Polymer Data Handbook. Oxford University Press, Inc. pp. 252–253. ISBN 978-0195107890.
- Kaiser, Nancy; Klein, Dan; Karanja, Peter; Greten, Zachariah; Newman, Jerry (2009). "Inactivation of chlorhexidine gluconate on skin by incompatible alcohol hand sanitizing gels". American Journal of Infection Control. 37 (7): 569–73. doi:10.1016/j.ajic.2008.12.008. PMID 19398245.
- Todica, M.; et al. (2015). "UV-Vis fluorescence investigation of some poly (acrylic) gels" (PDF). Studia UBB Chemia. 1: 7–17. Archived from the original (PDF) on 2015-12-25.
- Todica, M.; et al. (2015). "Spectroscopic investigation of some poly-(acrylic acid) gels with embedded gold nanoparticles" (PDF). Studia UBB Chemia. 1: 19–28. Archived from the original (PDF) on 2015-12-25.
