Food energy
Food energy is chemical energy that animals (including humans) derive from food through the process of cellular respiration. Cellular respiration may either involve the chemical reaction of food molecules with molecular oxygen[1] (aerobic respiration) or the process of reorganizing the food molecules without additional oxygen (anaerobic respiration).

Overview
Humans and other animals need a minimum intake of food energy to sustain their metabolism and to drive their muscles. Foods are composed chiefly of carbohydrates, fats, proteins, water, vitamins, and minerals. Carbohydrates, fats, proteins, and water represent virtually all the weight of food, with vitamins and minerals making up only a small percentage of the weight. (Carbohydrates, fats, and proteins comprise ninety percent of the dry weight of foods.[2]) Organisms derive food energy from carbohydrates, fats and proteins as well as from organic acids, polyols, and ethanol present in the diet.[3] Some diet components that provide little or no food energy, such as water, minerals, vitamins, cholesterol and insoluble fibre, may still be necessary to health and survival for other reasons. Water, minerals, vitamins, and cholesterol are not broken down (they are used by the body in the form in which they are absorbed) and so cannot be used for energy. Fibre cannot be completely digested by most animals, including humans, who can only extract 8.4 kJ/g (2 kcal/g) of food energy from it. Ruminants can extract nearly 17 kJ/g (4 kcal/g) from fibre with the aid of bacteria in their rumens.
Using the International System of Units, researchers measure energy in joules (J) or in its multiples; the kilojoule (kJ) is most often used for food-related quantities. An older metric system unit of energy, still widely used in food-related contexts, is the calorie; more precisely, the "food calorie", "large calorie" or kilocalorie (kcal or Cal), equal to 4184 joules. (Contrast the "small calorie" (cal), equal to 1/1000 of a food calorie, that is often used in chemistry and in physics.) Within the European Union, both the kilocalorie ("kcal") and kilojoule ("kJ") appear on nutrition labels. In many countries, only one of the units is displayed; in Canada and the United States labels spell out the unit as "calorie" or as "Calorie".
Fats and ethanol have the greatest amount of food energy per gram, 37 and 29 kilojoules per gram (8.8 and 6.9 kcal/g), respectively. Proteins and most carbohydrates both have about 17 kJ/g (4 kcal/g).[note 1] The differing energy density of foods (fat, alcohols, carbohydrates and proteins) lies mainly in their varying proportions of carbon, hydrogen, and oxygen atoms.[1] Carbohydrates that are not easily absorbed, such as fibre, or lactose in lactose-intolerant individuals, contribute less food energy. Polyols (including sugar alcohols) and organic acids contribute 10 kJ/g (2.4 kcal/g) and 13 kJ/g (3.1 kcal/g) respectively.[4]
Measure
Conventional food energy is based on heats of combustion in a bomb calorimeter and corrections that take into consideration the efficiency of digestion and absorption and the production of urea and other substances in the urine. The American chemist Wilbur Atwater worked these corrections out in the late 19th century[5] (see Atwater system for more detail). Based on the work of Atwater, it became common practice to calculate energy content of foods using 17 kJ/g (4 kcal/g) for carbohydrates and proteins and 38 kJ/g (9 kcal/g) for lipids.[5] The system was later improved by Annabel Merrill and Bernice Watt of the United States Department of Agriculture, who derived a system whereby specific calorie conversion factors for different foods were proposed.[6]
Nutrition labels
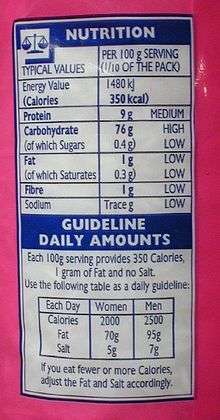
Many governments require food manufacturers to label the energy content of their products, to help consumers control their energy intake.[7] In the European Union, manufacturers of packaged food must label the nutritional energy of their products in both kilocalories and kilojoules, when required. In the United States, the equivalent mandatory labels display only "Calories" (kilocalories),[8] often as a substitute for the name of the quantity being measured, food energy; an additional kilojoules figure is optional and is rarely used. In Australia and New Zealand, the food energy must be stated in kilojoules (and optionally in kilocalories as well), and other nutritional energy information is similarly conveyed in kilojoules.[9][10] The energy available from the respiration of food is usually given on labels for 100 g, for a typical serving size (according to the manufacturer), and/or for the entire pack contents.
The amount of food energy associated with a particular food could be measured by completely burning the dried food in a bomb calorimeter, a method known as direct calorimetry.[11] However, the values given on food labels are not determined in this way. The reason for this is that direct calorimetry also burns the dietary fibre, and so does not allow for faecal losses; thus direct calorimetry would give systematic overestimates of the amount of fuel that actually enters the blood through digestion. What are used instead are standardized chemical tests or an analysis of the recipe using reference tables for common ingredients[12] to estimate the product's digestible constituents (protein, carbohydrate, fat, etc.). These results are then converted into an equivalent energy value based on the following standardized table of energy densities.[4][13] However "energy density" is a misleading term for it once again assumes that energy is IN the particular food, whereas it simply means that "high density" food needs more oxygen during respiration, leading to greater transfer of energy.[1][note 2]
Note that the following standardized table of energy densities[13] is an approximation and the value in kJ/g does not convert exactly to kcal/g using a conversion factor.
The use of such a simple system has been criticized for not taking into consideration other factors pertaining to the influence of different foods on obesity.[5]
| Food component | Energy density[14] | |
|---|---|---|
| kJ/g | kcal/g | |
| Fat | 37 | 9 |
| Ethanol (drinking alcohol) | 29 | 7 |
| Proteins | 17 | 4 |
| Carbohydrates | 17 | 4 |
| Organic acids | 13 | 3 |
| Polyols (sugar alcohols, sweeteners) | 10 | 2.4 |
| Fibre | 8 | 2 |
All the other nutrients in food are noncaloric and are thus not counted.
Recommended daily intake
Increased mental activity has been linked with moderately increased brain energy consumption.[15] Older people and those with sedentary lifestyles require less energy; children and physically active people require more.
According to the Food and Agriculture Organization of the United Nations, the average minimum energy requirement per person per day is about 7,500 kJ (1,800 kcal).[16]
Recommendations in the United States are 10,900 and 8,400 kJ (2,600 and 2,000 kcal) for men and women (respectively) between 31 and 35, at a physical activity level equivalent to walking about 2 to 5 km (1 1⁄2 to 3 mi) per day at 5 to 6 km/h (3 to 4 mph) in addition to the light physical activity associated with typical day-to-day life.[17] French guidance suggests roughly the same levels.[18]
For young children, estimated caloric needs range from 4,200 to 8,400 kilojoules (1,000 to 2,000 kcal) per day. The recommended caloric intake for older children and adolescents, on the other hand, varies greatly from 1,400 to 3,200 kilocalories (5,900 to 13,400 kJ) per day. Boys in general require higher caloric intake than girls.[17]
Recognizing that people of different age and gender groups have varying daily activity levels, Australia's National Health and Medical Research Council recommends no single daily energy intake but instead prescribes an appropriate recommendation for each age and gender group.[19] Notwithstanding, nutrition labels on Australian food products typically recommend the average daily energy intake of 8,800 kJ (2,100 kcal).
Energy usage in the human body
The human body uses the energy released by respiration for a range of purposes: about 20% of the energy is used for brain metabolism, much is used for the basal metabolic requirements of other organs and tissues, and a proportion is used for the production of mechanical energy by skeletal muscle to maintain posture and produce motion.
The efficiency of muscles is low: only 18 to 26% of the energy available from food is converted into mechanical energy.[20] This low efficiency is the result of about 40% efficiency for generating ATP from the respiration of food, losses in converting energy from ATP into mechanical work inside the muscle, and mechanical losses inside the body. For an overall efficiency of 20%, one watt of mechanical power is equivalent to 18 kJ (4.3 kcal) per hour. For example, one manufacturer of rowing equipment shows calories used from 'burning' food as four times the actual mechanical work plus 1,300 kJ (300 kcal) per hour,[21] which amounts to about 20% efficiency at 250 watts of mechanical output.
Changes in body temperature – either hotter or cooler – increase the metabolic rate, thus burning more energy. Prolonged exposure to extremely warm or very cold environments increases the basal metabolic rate (BMR). People who live in these types of settings often have BMRs 5–20% higher than those in other climates.
See also
Notes
- The heats of combustion for glucose, sucrose, and starch are 15.57, 16.48 and 17.48 kilojoules per gram (3.72, 3.94 and 4.18 kcal/g) respectively.
- See, for example, the Energy section (follow "Fuels") in Science Issues.
References
- Schmidt-Rohr K (2015). "Why Combustions Are Always Exothermic, Yielding About 418 kJ per Mole of O2". J. Chem. Educ. 92 (12): 2094–2099. Bibcode:2015JChEd..92.2094S. doi:10.1021/acs.jchemed.5b00333.
- Youdim, Adrienne. "Carbohydrates, Proteins, and Fats". Merck Manual: Consumer Version. Merck & Co., Inc. Retrieved 13 December 2019.
- Littledyke, Michael; Ross, Keith; Lakin, Liz (2000). "Energy and fuels". Science Knowledge and the Environment: A Guide for Students and Teachers in Primary Education (1st ed.). London: David Fulton. pp. 78–95. ISBN 1-85346-625-5.
- "Schedule 7: Nutrition labelling". Legislation.gov.uk. The National Archives. 1 July 1996. Retrieved 13 December 2019.
- Bijal Trivedi (15 July 2009). "The calorie delusion: Why food labels are wrong". New Scientist.
- Annabel Merrill; Bernice Watt (1973). Energy Values of Food ... basis and derivation (PDF). United States Department of Agriculture. Archived (PDF) from the original on 22 November 2016.
- "Nutrition labelling (until 2014)". EUR-Lex. Publications Office of the European Union. 24 September 1990. Retrieved 13 December 2019.
- "Section 101.9 - Nutrition labeling of food". govinfo. U.S. Government Publishing Office. 1 April 2004. Retrieved 13 December 2019.
- "Nutrition information panels". Food Standards Australia & New Zealand. December 2015. Retrieved 13 December 2019.
- "8700 – Find your ideal figure". 8700.com.au. NSW Health. Retrieved 12 December 2019.
- Youdim, Adrienne. "Calories". Merck Manual: Consumer Version. Merck & Co., Inc. Retrieved 13 December 2019.
- Nutrient Value of Some Common Foods. Health Canada. 2008. p. 4. ISBN 978-0-662-48082-2. Retrieved 13 December 2019.
- "Council directive 90/496/EEC of 24 September 1990 on nutrition labelling for foodstuffs". EUR-Lex. Publications Office of the European Union. 24 September 1990. Retrieved 11 June 2018.
- "Chapter 3: Calculation Of The Energy Content Of Foods – Energy Conversion Factors". Food and Agriculture Organization of the United Nations. Retrieved 30 March 2017.
- Larsen, Gerald; Haier, Richard; LaCasse, Lori; Hazen, Kay (November–December 1995). "Evaluation of a "mental effort" hypothesis for correlations between cortical metabolism and intelligence". Intelligence. 21 (3): 267–278. doi:10.1016/0160-2896(95)90017-9.
- "Hunger and food insecurity". Food and Agriculture Organization of the United Nations. Retrieved 13 December 2019.
- "Appendix 2. Estimated Calorie Needs per Day, by Age, Sex, and Physical Activity Level – 2015–2020 Dietary Guidelines". Office of Disease Prevention and Health Promotion. U.S. Department of Health and Human Services and U.S. Department of Agriculture. December 2015. Retrieved 13 December 2019.
- "Recommended energy intake" (PDF) (in French). French Agency for Food, Environmental and Occupational Health & Safety. Archived from the original (PDF) on 26 November 2013. Retrieved 30 April 2014.
- "Dietary Energy". Nutrient Reference Values for Australia and New Zealand. National Health and Medical Research Council. 2014-03-13. Retrieved 13 December 2019.
- Seiler, Stephen (1996). "Efficiency, Economy and Endurance Performance". Archived from the original on 21 December 2007. Retrieved 13 December 2019.
- "Concept II Rowing Ergometer – Use Guide" (PDF). Concept2. Concept II, Inc. Spring 1993. Archived from the original (PDF) on 26 December 2010. Retrieved 13 December 2019.
.svg.png)