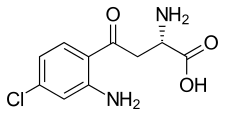
4-Chlorokynurenine
L-4-Chlorokynurenine (4-Cl-KYN; developmental code name AV-101) is an orally active small molecule prodrug of 7-chlorokynurenic acid, a NMDA receptor antagonist. It appears to be a rapid-acting antidepressant.
 | |
 | |
| Clinical data | |
|---|---|
| Other names | 4-Cl-KYN; AV-101; 3-(4-Chloroanthraniloyl)-DL-alanine |
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code |
|
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 39–84% (rodents); ≥ 31% (humans) |
| Elimination half-life | 2–3 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C10H11ClN2O3 |
| Molar mass | 242.66 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
AV-101 was discovered at Marion Merrell Dow and its biological activity was explored at University of Maryland. It underwent initial development at Artemis Neuroscience which was acquired by VistaGen in 2003. As of 2016 it was in a Phase II clinical trial for major depressive disorder.
Pharmacology

4-Chlorokynurenine penetrates the blood–brain barrier via the large neutral amino acid transporter 1.[2] In the central nervous system it is converted to 7-chlorokynurenic acid by kynurenine aminotransferase in astrocytes.[3]
Most of its therapeutic potential is believed to occur via 7-chlorokynurenic acid which inhibits the glycine co-agonist site of NMDA receptors.[3]
Another metabolite, 4-chloro-3-hydroxy-anthranilic acid, inhibits the enzyme 3-hydroxyanthranilate oxidase, which provides a rationale for further testing in neurodegenerative diseases.[3]
Chemistry
4-Chlorokynurenine is prodrug of 7-chlorokynurenic acid (7-Cl-KYNA), which in turn is a halogenated derivative of L-kynurenine.[3]
History
Artemis Neuroscience was formed to develop work done by University of Maryland professor Robert Schwartz in collaboration with scientists at Marion Merrell Dow (which became part of Sanofi by way of Aventis); this work included AV-101.[4][5][6]
VistaGen acquired AV-101 when it acquired Artemis in 2003.[7]
VistaGen filed an Investigational New Drug application with the FDA for use of AV-101 in neuropathic pain in 2013.[3]
In 2013, other NMDA receptor antagonists in clinical trials for depression included lanicemine, esketamine, and rapastinel, with lanicemine being the most advanced.[8]
Research
AV-101 showed efficacy in animal model of Huntington's disease[3] and showed rapid-acting antidepressant effects similar to ketamine in the forced-swim test and two other behavioral models of depression in rodents.[9]
By 2013 AV-101 had successfully gone through two Phase I clinical trials.[3]
As of 2016 a Phase II clinical trial was underway in major depressive disorder.[9]
By 2019 a phase II clinical trial had shown negative results for major depressive disorder.[10]
It has been found to be effective for neuropathic pain in animal studies. [11]
References
- Laube B, Hirai H, Sturgess M, Betz H, Kuhse J (1997). "Molecular determinants of agonist discrimination by NMDA receptor subunits: analysis of the glutamate binding site on the NR2B subunit". Neuron. 18 (3): 493–503. doi:10.1016/S0896-6273(00)81249-0. PMID 9115742.
- Smith, Quentin R.; Lockman, Paul R. (2011). "11. Prodrug Approaches for Central Nervous System Delivery". In Mannhold, R; Kubinyi, H; Folkers, G (eds.). Prodrugs and Targeted Delivery: Towards Better ADME Properties Volume 47 of Methods and Principles in Medicinal Chemistry. John Wiley & Sons. p. 259. ISBN 9783527633180.
- Vécsei, L; Szalárdy, L; Fülöp, F; Toldi, J (January 2013). "Kynurenines in the CNS: recent advances and new questions". Nature Reviews. Drug Discovery. 12 (1): 64–82. doi:10.1038/nrd3793. PMID 23237916.
- "School of Medicine Professor Wins University System of Maryland (USM) Board of Regents Award". University of Maryland. April 6, 2007. Archived from the original on January 13, 2017. Retrieved January 12, 2017.
- "Press Release: VistaGen Therapeutics Acquires Artemis Neuroscience, Inc. - Enters Late-Stage Preclinical Development Program for Lead Epilepsy Drug Candidate -". PR Newswire. November 19, 2003.
- "VistaGen Therapeutics, Inc. 8-K Exhibit 10-26". SEC Edgar. May 16, 2011. See 8-K Index page at SEC Edgar.
- "VistaGen acquires Artemis Neuroscience". San Francisco Business Times. November 19, 2003.
- Flight, Monica Hoyos (29 November 2013). "Trial watch: Phase II boost for glutamate-targeted antidepressants". Nature Reviews Drug Discovery. 12 (12): 897. doi:10.1038/nrd4178. PMID 24287771.
- Gerhard, DM; Wohleb, ES; Duman, RS (March 2016). "Emerging treatment mechanisms for depression: focus on glutamate and synaptic plasticity". Drug Discovery Today. 21 (3): 454–64. doi:10.1016/j.drudis.2016.01.016. PMC 4803609. PMID 26854424.
- Hashimoto, Kenji (2019). "Rapid‐acting antidepressant ketamine, its metabolites and other candidates: A historical overview and future perspective". Psychiatry and Clinical Neurosciences. 73 (10): 613–627. doi:10.1111/pcn.12902. ISSN 1323-1316. PMC 6851782. PMID 31215725.
- Yaksh, Tony L.; Schwarcz, Robert; Snodgrass, H. Ralph (October 2017). "Characterization of the Effects of L-4-Chlorokynurenine on Nociception in Rodents". The Journal of Pain. 18 (10): 1184–1196. doi:10.1016/j.jpain.2017.03.014. ISSN 1526-5900. PMID 28428091.
External links
- AV-101 - AdisInsight
- AV-101 - A Potential Breakthrough in Depression Treatment - VistaGen Therapeutics - YouTube