ACPD
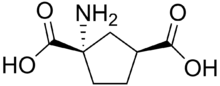
1-Amino-1,3-dicarboxycyclopentane (ACPD) is a chemical compound that binds to the metabotropic glutamate receptor (mGluR),[2] acting as a mGluR agonist. ACPD is a rigid analogue of the neurotransmitter glutamate and does not activate ionotropic glutamate receptors.[3] However, it has been reported to be an agonist of the glycine site of the NMDA receptor. ACPD can induce convulsions in neonatal rats.[4]
 | |
| Names | |
|---|---|
| Systematic IUPAC name
1-Aminocyclopentane-1,3-dicarboxylic acid[1] | |
| Identifiers | |
| |
3D model (JSmol) |
|
| Abbreviations | ACPD |
| ChEMBL | |
| ChemSpider | |
| MeSH | 1-amino-1,3-dicarboxycyclopentane |
PubChem CID |
|
| RTECS number |
|
| |
| |
| Properties | |
| C7H11NO4 | |
| Molar mass | 173.168 g·mol−1 |
| Appearance | White crystals |
| 20 g dm−3 | |
| Solubility in ethanol | 240 mg dm−3 |
| log P | -0.709 |
| Acidity (pKa) | 2.112 |
| Basicity (pKb) | 11.885 |
| Isoelectric point | 2.84 |
| Hazards | |
| GHS pictograms |  |
| GHS Signal word | Warning |
GHS hazard statements |
H302, H312, H315, H319, H332, H335 |
| P261, P280, P305+351+338 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
References
- "1-amino-1,3-dicarboxycyclopentane - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 25 March 2005. Identification and Related Records. Retrieved 15 October 2011.
- Schoepp DD, True RA (September 1992). "1S,3R-ACPD-sensitive (metabotropic) [3H]glutamate receptor binding in membranes". Neurosci. Lett. 145 (1): 100–4. doi:10.1016/0304-3940(92)90213-Q. PMID 1461560.
- Manzoni O, Fagni L, Pin JP, Rassendren F, Poulat F, Sladeczek F, Bockaert J (July 1990). "(trans)-1-amino-cyclopentyl-1,3-dicarboxylate stimulates quisqualate phosphoinositide-coupled receptors but not ionotropic glutamate receptors in striatal neurons and Xenopus oocytes". Mol. Pharmacol. 38 (1): 1–6. PMID 2164627.
- McDonald JW, Fix AS, Tizzano JP, Schoepp DD (October 1993). "Seizures and brain injury in neonatal rats induced by 1S,3R-ACPD, a metabotropic glutamate receptor agonist". J. Neurosci. 13 (10): 4445–55. doi:10.1523/JNEUROSCI.13-10-04445.1993. PMC 6576384. PMID 8410197.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.