Apimostinel
Apimostinel (former developmental code name NRX-1074) is an antidepressant, acting as a selective partial agonist of an allosteric site of the glycine site of the NMDA receptor complex, which is under investigation by Naurex and Allergan for the treatment of major depressive disorder (MDD).[1][2][3][4][5] As of 2015, an intravenous formulation of apimostinel is in a phase II clinical trial for MDD,[3][6] and an oral formulation is concurrently in phase I trials for MDD.
 | |
| Clinical data | |
|---|---|
| Other names | NRX-1074; AGN-241660; Threonyl-prolyl-2R-(2-benzyl)-prolyl-threonine amide |
| Routes of administration | By mouth |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
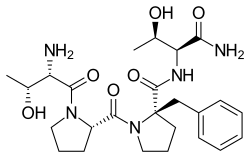
| Formula | C25H37N5O6 |
| Molar mass | 503.600 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Its mechanism of action and effects are similar to those of rapastinel (GLYX-13), which is under development as an adjunctive therapy for treatment-resistant depression also by Naurex. However, apimostinel is 100-fold more potent by weight and, whereas rapastinel must be administered via intravenous injection, is orally-active.[3] Apimostinel is intended by Naurex as an improved, follow-up drug to rapastinel. Similarly to rapastinel, apimostinel is an amidated tetrapeptide, and has almost an identical chemical structure to rapastinel, but has been structurally modified via the addition of a benzyl group. The drug has shown rapid antidepressant effects in pre-clinical models of depression.[3] In addition, similarly to rapastinel, it is well tolerated and lacks the schizophrenia-like psychotomimetic effects of other NMDA receptor antagonists such as ketamine.[3]
Commercial competitors of apimostinel include AV-101 from VistaGen Therapeutics and Cerecor's CERC-301.[8]
References
- Hayley S, Litteljohn D (2013). "Neuroplasticity and the next wave of antidepressant strategies". Front Cell Neurosci. 7: 218. doi:10.3389/fncel.2013.00218. PMC 3834236. PMID 24312008.
Despite the mounting evidence indicating that ketamine has rapid and robust antidepressant properties (and notwithstanding the earlier mentioned preliminary clinical data indicating that long-term, low-dose ketamine may be both tolerable and effective; e.g., Messer et al., 2010), concerns over ketamine’s psychotomimetic effects have spurred intensive efforts to develop safer and more tolerable glutamate-based antidepressants. At the vanguard of this movement are the “next generation” NMDA receptor antagonists. Included here are the aminoadamantanes, memantine and amantadine (Sani et al., 2012); the NR2B-selective antagonists, traxoprodil (CP-101,606; Preskorn et al., 2008) and MK-0657 (Ibrahim et al., 2012a); and the low-affinity NMDA channel blocker AZD6765 (Zarate et al., 2013). The NMDA receptor glycine-site functional partial agonist, GLYX-13, and its orally bioavailable and presumed more potent analog, NRX-1074, have also garnered the recent attention of researchers and clinicians (Burgdorf et al., 2013; Dolgin, 2013), as have several modulators of metabotropic glutamate receptors (e.g., the mGluR7 allosteric agonist AMN082; Bradley et al., 2012) and select α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor potentiators (e.g., Org 26576; Nations et al., 2012).
- PR Newswire (2010). "Naurex's Novel Antidepressant GLYX-13 Recognized as One of Windhover's Top 10 Neuroscience Projects to Watch".
- PR Newswire (2014). "Naurex Reports Positive Top-Line Phase 2b Results for Novel Antidepressant GLYX-13 and Advances NRX-1074 into Phase 2 Depression Study".
- Dang YH, Ma XC, Zhang JC, et al. (January 2014). "Targeting of NMDA Receptors in the Treatment of Major Depression". Curr. Pharm. Des. 20 (32): 5151–9. doi:10.2174/1381612819666140110120435. PMID 24410564.
- plc, Allergan. "Allergan Successfully Completes Naurex Acquisition". www.prnewswire.com. Retrieved 2016-11-20.
- "Study of Intravenous NRX-1074 in Patients With Major Depressive Disorder". Clinicaltrials.gov. US National Institutes of Health. Retrieved 10 December 2014.