Taiga
Taiga (/ˈtaɪɡə/; Russian: тайга́, IPA: [tɐjˈɡa]; relates to Mongolic[1] and Turkic[2] languages), generally referred to in North America as boreal forest or snow forest, is a biome characterized by coniferous forests consisting mostly of pines, spruces, and larches.
| Taiga | |
|---|---|
 | |
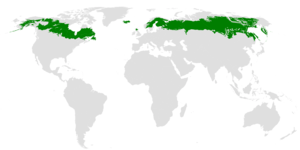 The taiga is found throughout the high northern latitudes, between the tundra and the temperate forest, from about 50°N to 70°N, but with considerable regional variation. | |
| Ecology | |
| Biome | Terrestrial subarctic, humid |
| Geography | |
| Countries | Russia, Mongolia, Japan, Norway, Sweden, Iceland, Finland, United States, Canada, Scotland (United Kingdom) |
| Climate type | Dfc, Dwc, Dsc, Dfd, Dwd, Dsd |
The taiga or boreal forest is the world's largest land biome.[3] In North America, it covers most of inland Canada, Alaska, and parts of the northern contiguous United States.[4] In Eurasia, it covers most of Sweden, Finland, much of Russia from Karelia in the west to the Pacific Ocean (including much of Siberia), much of Norway and Estonia, some of the Scottish Highlands, some lowland/coastal areas of Iceland, and areas of northern Kazakhstan, northern Mongolia, and northern Japan (on the island of Hokkaidō). The main tree species, the length of the growing season and summer temperatures vary. For example, the taiga of North America mostly consists of spruces; Scandinavian and Finnish taiga consists of a mix of spruce, pines and birch; Russian taiga has spruces, pines and larches depending on the region, while the Eastern Siberian taiga is a vast larch forest. The Taiga in its current form is a relatively recent phenomenon, having only existed for the last 12,000 years since the beginning of the Holocene epoch, covering land that had been mammoth steppe or under the Scandinavian Ice Sheet in Eurasia and under the Laurentide Ice Sheet in North America during the Late Pleistocene.
A different use of the term taiga is often encountered in the English language, with "boreal forest" used in the United States and Canada to refer to only the more southerly part of the biome, while "taiga" is used to describe the more barren areas of the northernmost part of the biome approaching the tree line and the tundra biome. Hoffman (1958) discusses the origin of this differential use in North America and why it is an inappropriate differentiation of the Russian term. Although at high elevations taiga grades into alpine tundra through Krummholz, it is not exclusively an alpine biome; and unlike subalpine forest, much of taiga is lowlands.

Climate and geography
Taiga is the world's largest land biome [3] (depending on how one defines a biome, it could also be considered the second-largest, after deserts and xeric shrublands), covering 17 million square kilometres (6.6 million square miles) or 11.5% of the Earth's land area.[5] The largest areas are located in Russia and Canada. The taiga is the terrestrial biome with the lowest annual average temperatures after the tundra and permanent ice caps. Extreme winter minimums in the northern taiga are typically lower than those of the tundra. The lowest reliably recorded temperatures in the Northern Hemisphere were recorded in the taiga of northeastern Russia. The taiga or boreal forest has a subarctic climate with very large temperature range between seasons, but the long and cold winter is the dominant feature. This climate is classified as Dfc, Dwc, Dsc, Dfd and Dwd in the Köppen climate classification scheme,[6] meaning that the short summer (24 h average 10 °C (50 °F) or more) lasts 1–3 months and always less than 4 months. In Siberian taiga the average temperature of the coldest month is between −6 °C (21 °F) and −50 °C (−58 °F).[7] There are also some much smaller areas grading towards the oceanic Cfc climate with milder winters, whilst the extreme south and (in Eurasia) west of the taiga reaches into humid continental climates (Dfb, Dwb) with longer summers. The mean annual temperature generally varies from −5 to 5 °C (23 to 41 °F),[8] but there are taiga areas in eastern Siberia and interior Alaska-Yukon where the mean annual reaches down to −10 °C (14 °F).[9][10] According to some sources, the boreal forest grades into a temperate mixed forest when mean annual temperature reaches about 3 °C (37 °F).[11] Discontinuous permafrost is found in areas with mean annual temperature below freezing (0 °C; 32 °F), whilst in the Dfd and Dwd climate zones continuous permafrost occurs and restricts growth to very shallow-rooted trees like Siberian larch. The winters, with average temperatures below freezing, last five to seven months. Temperatures vary from −54 to 30 °C (−65 to 86 °F) throughout the whole year. The summers, while short, are generally warm and humid. In much of the taiga, −20 °C (−4 °F) would be a typical winter day temperature and 18 °C (64 °F) an average summer day.

The growing season, when the vegetation in the taiga comes alive, is usually slightly longer than the climatic definition of summer as the plants of the boreal biome have a lower threshold to trigger growth. In Canada, Scandinavia and Finland, the growing season is often estimated by using the period of the year when the 24-hour average temperature is +5 °C (41 °F) or more.[12] For the Taiga Plains in Canada, growing season varies from 80 to 150 days, and in the Taiga Shield from 100 to 140 days.[13] Some sources claim 130 days growing season as typical for the taiga.[3] Other sources mention that 50–100 frost-free days are characteristic.[14] Data for locations in southwest Yukon gives 80–120 frost-free days.[15] The closed canopy boreal forest in Kenozersky National Park near Plesetsk, Arkhangelsk Province, Russia, on average has 108 frost-free days.[16] The longest growing season is found in the smaller areas with oceanic influences; in coastal areas of Scandinavia and Finland, the growing season of the closed boreal forest can be 145–180 days.[17] The shortest growing season is found at the northern taiga–tundra ecotone, where the northern taiga forest no longer can grow and the tundra dominates the landscape when the growing season is down to 50–70 days,[18][19] and the 24-hr average of the warmest month of the year usually is 10 °C (50 °F) or less.[20] High latitudes mean that the sun does not rise far above the horizon, and less solar energy is received than further south. But the high latitude also ensures very long summer days, as the sun stays above the horizon nearly 20 hours each day, or up to 24 hours, with only around 6 hours of daylight, or none, occurring in the dark winters, depending on latitude. The areas of the taiga inside the Arctic Circle have midnight sun in mid-summer and polar night in mid-winter.
The taiga experiences relatively low precipitation throughout the year (generally 200–750 mm (7.9–29.5 in) annually, 1,000 mm (39 in) in some areas), primarily as rain during the summer months, but also as fog and snow. This fog, especially predominant in low-lying areas during and after the thawing of frozen Arctic seas, means that sunshine is not abundant in the affected taiga areas even during the long summer days. As evaporation is consequently low for most of the year, precipitation exceeds evaporation, and is sufficient to sustain the dense vegetation growth including large trees. (In the steppe biome, often found south of taiga in the northern hemisphere, evapotranspiration exceeds precipitation, restricting vegetation to mostly grasses.)
Snow may remain on the ground for as long as nine months in the northernmost extensions of the taiga biome.[23]
In general, taiga grows to the south of the 10 °C (50 °F) July isotherm, but occasionally as far north as the 9 °C (48 °F) July isotherm.[24] Rich in spruces, Scots pines in the western Siberian plain, the taiga is dominated by larch in Eastern Siberia, before returning to its original floristic richness on the Pacific shores. Two deciduous trees mingle throughout southern Siberia: birch and Populus tremula.[7]
The southern limit is more variable, depending on rainfall; taiga may be replaced by forest steppe south of the 15 °C (59 °F) July isotherm where rainfall is very low, but more typically extends south to the 18 °C (64 °F) July isotherm, and locally where rainfall is higher (notably in eastern Siberia and adjacent Outer Manchuria) south to the 20 °C (68 °F) July isotherm. In these warmer areas the taiga has higher species diversity, with more warmth-loving species such as Korean pine, Jezo spruce, and Manchurian fir, and merges gradually into mixed temperate forest or, more locally (on the Pacific Ocean coasts of North America and Asia), into coniferous temperate rainforests where oak and hornbeam appear and join the conifers, birch and Populus tremula.
The area currently classified as taiga in Europe and North America (except Alaska) was recently glaciated. As the glaciers receded they left depressions in the topography that have since filled with water, creating lakes and bogs (especially muskeg soil) found throughout the taiga.

In Sweden the taiga is associated with the Norrland terrain.[25]
Soils
._%D0%9F%D0%BE%D0%BB%D1%8F_%D1%82%D1%83%D0%BA%D1%83%D0%BB%D0%B0%D0%BD%D0%BE%D0%B2_(%D0%BF%D0%B5%D1%81%D1%87%D0%B0%D0%BD%D1%8B%D0%B5_%D0%B4%D1%8E%D0%BD%D1%8B)._(10118322213).jpg)
Taiga soil tends to be young and poor in nutrients. It lacks the deep, organically enriched profile present in temperate deciduous forests.[26] The thinness of the soil is due largely to the cold, which hinders the development of soil and the ease with which plants can use its nutrients.[26] Fallen leaves and moss can remain on the forest floor for a long time in the cool, moist climate, which limits their organic contribution to the soil; acids from evergreen needles further leach the soil, creating spodosol, also known as podzol.[27] Since the soil is acidic due to the falling pine needles, the forest floor has only lichens and some mosses growing on it. In clearings in the forest and in areas with more boreal deciduous trees, there are more herbs and berries growing. Diversity of soil organisms in the boreal forest is high, comparable to the tropical rainforest.[28]
Flora
.jpg)
Since North America and Asia used to be connected by the Bering land bridge, a number of animal and plant species (more animals than plants) were able to colonize both continents and are distributed throughout the taiga biome (see Circumboreal Region). Others differ regionally, typically with each genus having several distinct species, each occupying different regions of the taiga. Taigas also have some small-leaved deciduous trees like birch, alder, willow, and poplar; mostly in areas escaping the most extreme winter cold. However, the Dahurian larch tolerates the coldest winters in the Northern Hemisphere in eastern Siberia. The very southernmost parts of the taiga may have trees such as oak, maple, elm and lime scattered among the conifers, and there is usually a gradual transition into a temperate mixed forest, such as the eastern forest-boreal transition of eastern Canada. In the interior of the continents with the driest climate, the boreal forests might grade into temperate grassland.
There are two major types of taiga. The southern part is the closed canopy forest, consisting of many closely spaced trees with mossy ground cover. In clearings in the forest, shrubs and wildflowers are common, such as the fireweed. The other type is the lichen woodland or sparse taiga, with trees that are farther-spaced and lichen ground cover; the latter is common in the northernmost taiga.[29] In the northernmost taiga the forest cover is not only more sparse, but often stunted in growth form; moreover, ice pruned asymmetric black spruce (in North America) are often seen, with diminished foliage on the windward side.[30] In Canada, Scandinavia and Finland, the boreal forest is usually divided into three subzones: The high boreal (north boreal) or taiga zone; the middle boreal (closed forest); and the southern boreal, a closed canopy boreal forest with some scattered temperate deciduous trees among the conifers,[31] such as maple, elm and oak. This southern boreal forest experiences the longest and warmest growing season of the biome, and in some regions (including Scandinavia, Finland and western Russia) this subzone is commonly used for agricultural purposes. The boreal forest is home to many types of berries; some are confined to the southern and middle closed boreal forest (such as wild strawberry and partridgeberry); others grow in most areas of the taiga (such as cranberry and cloudberry), and some can grow in both the taiga and the low arctic (southern part of) tundra (such as bilberry, bunchberry and lingonberry).
The forests of the taiga are largely coniferous, dominated by larch, spruce, fir and pine. The woodland mix varies according to geography and climate so for example the Eastern Canadian forests ecoregion of the higher elevations of the Laurentian Mountains and the northern Appalachian Mountains in Canada is dominated by balsam fir Abies balsamea, while further north the Eastern Canadian Shield taiga of northern Quebec and Labrador is notably black spruce Picea mariana and tamarack larch Larix laricina.
Evergreen species in the taiga (spruce, fir, and pine) have a number of adaptations specifically for survival in harsh taiga winters, although larch, which is extremely cold-tolerant,[32] is deciduous. Taiga trees tend to have shallow roots to take advantage of the thin soils, while many of them seasonally alter their biochemistry to make them more resistant to freezing, called "hardening".[33] The narrow conical shape of northern conifers, and their downward-drooping limbs, also help them shed snow.[33]
Because the sun is low in the horizon for most of the year, it is difficult for plants to generate energy from photosynthesis. Pine, spruce and fir do not lose their leaves seasonally and are able to photosynthesize with their older leaves in late winter and spring when light is good but temperatures are still too low for new growth to commence. The adaptation of evergreen needles limits the water lost due to transpiration and their dark green color increases their absorption of sunlight. Although precipitation is not a limiting factor, the ground freezes during the winter months and plant roots are unable to absorb water, so desiccation can be a severe problem in late winter for evergreens.

Although the taiga is dominated by coniferous forests, some broadleaf trees also occur, notably birch, aspen, willow, and rowan. Many smaller herbaceous plants, such as ferns and occasionally ramps grow closer to the ground. Periodic stand-replacing wildfires (with return times of between 20–200 years) clear out the tree canopies, allowing sunlight to invigorate new growth on the forest floor. For some species, wildfires are a necessary part of the life cycle in the taiga; some, e.g. jack pine have cones which only open to release their seed after a fire, dispersing their seeds onto the newly cleared ground; certain species of fungi (such as morels) are also known to do this. Grasses grow wherever they can find a patch of sun, and mosses and lichens thrive on the damp ground and on the sides of tree trunks. In comparison with other biomes, however, the taiga has low biological diversity.

Coniferous trees are the dominant plants of the taiga biome. A very few species in four main genera are found: the evergreen spruce, fir and pine, and the deciduous larch. In North America, one or two species of fir and one or two species of spruce are dominant. Across Scandinavia and western Russia, the Scots pine is a common component of the taiga, while taiga of the Russian Far East and Mongolia is dominated by larch.
Fauna

The boreal forest, or taiga, supports a relatively small range of animals due to the harshness of the climate. Canada's boreal forest includes 85 species of mammals, 130 species of fish, and an estimated 32,000 species of insects.[34] Insects play a critical role as pollinators, decomposers, and as a part of the food web. Many nesting birds rely on them for food in the summer months. The cold winters and short summers make the taiga a challenging biome for reptiles and amphibians, which depend on environmental conditions to regulate their body temperatures, and there are only a few species in the boreal forest including red-sided garter snake, common European adder, blue-spotted salamander, northern two-lined salamander, Siberian salamander, wood frog, northern leopard frog, boreal chorus frog, American toad, and Canadian toad. Most hibernate underground in winter. Fish of the taiga must be able to withstand cold water conditions and be able to adapt to life under ice-covered water. Species in the taiga include Alaska blackfish, northern pike, walleye, longnose sucker, white sucker, various species of cisco, lake whitefish, round whitefish, pygmy whitefish, Arctic lamprey, various grayling species, brook trout (including sea-run brook trout in the Hudson Bay area), chum salmon, Siberian taimen, lenok and lake chub.
The taiga is home to a number of large herbivorous mammals, such as moose and reindeer/caribou. Some areas of the more southern closed boreal forest also have populations of other deer species such as the elk (wapiti) and roe deer.[35][36] The largest animal in the taiga is the wood bison, found in northern Canada, Alaska and has been newly introduced into the Russian far-east.[37] Small mammals of the Taiga biome include rodent species including beaver, squirrel, North American porcupine and vole, as well as a small number of lagomorph species such as snowshoe hare and mountain hare. These species have adapted to survive the harsh winters in their native ranges. Some larger mammals, such as bears, eat heartily during the summer in order to gain weight, and then go into hibernation during the winter. Other animals have adapted layers of fur or feathers to insulate them from the cold. Predatory mammals of the taiga must be adapted to travel long distances in search of scattered prey or be able to supplement their diet with vegetation or other forms of food (such as raccoons). Mammalian predators of the taiga include Canada lynx, Eurasian lynx, stoat, Siberian weasel, least weasel, sable, American marten, North American river otter, European otter, American mink, wolverine, Asian badger, fisher, gray wolf, coyote, red fox, brown bear, American black bear, Asiatic black bear, polar bear (only small areas at the taiga – tundra ecotone) and Siberian tiger.
More than 300 species of birds have their nesting grounds in the taiga.[38] Siberian thrush, white-throated sparrow, and black-throated green warbler migrate to this habitat to take advantage of the long summer days and abundance of insects found around the numerous bogs and lakes. Of the 300 species of birds that summer in the taiga only 30 stay for the winter.[39] These are either carrion-feeding or large raptors that can take live mammal prey, including golden eagle, rough-legged buzzard (also known as the rough-legged hawk), and raven, or else seed-eating birds, including several species of grouse and crossbills.
Fire

Fire has been one of the most important factors shaping the composition and development of boreal forest stands (Rowe 1955);[40] it is the dominant stand-renewing disturbance through much of the Canadian boreal forest (Amiro et al. 2001).[41] The fire history that characterizes an ecosystem is its fire regime, which has 3 elements: (1) fire type and intensity (e.g., crown fires, severe surface fires, and light surface fires), (2) size of typical fires of significance, and (3) frequency or return intervals for specific land units.[42] The average time within a fire regime to burn an area equivalent to the total area of an ecosystem is its fire rotation (Heinselman 1973)[43] or fire cycle (Van Wagner 1978).[44] However, as Heinselman (1981) noted,[42] each physiographic site tends to have its own return interval, so that some areas are skipped for long periods, while others might burn two-times or more often during a nominal fire rotation.
The dominant fire regime in the boreal forest is high-intensity crown fires or severe surface fires of very large size, often more than 10,000 ha (100 km²), and sometimes more than 400,000 ha (4000 km²).[42] Such fires kill entire stands. Fire rotations in the drier regions of western Canada and Alaska average 50–100 years, shorter than in the moister climates of eastern Canada, where they may average 200 years or more. Fire cycles also tend to be long near the tree line in the subarctic spruce-lichen woodlands. The longest cycles, possibly 300 years, probably occur in the western boreal in floodplain white spruce.[42]
Amiro et al. (2001) calculated the mean fire cycle for the period 1980 to 1999 in the Canadian boreal forest (including taiga) at 126 years.[41] Increased fire activity has been predicted for western Canada, but parts of eastern Canada may experience less fire in future because of greater precipitation in a warmer climate.[45]
The mature boreal forest pattern in the south shows balsam fir dominant on well-drained sites in eastern Canada changing centrally and westward to a prominence of white spruce, with black spruce and tamarack forming the forests on peats, and with jack pine usually present on dry sites except in the extreme east, where it is absent.[46] The effects of fires are inextricably woven into the patterns of vegetation on the landscape, which in the east favour black spruce, paper birch, and jack pine over balsam fir, and in the west give the advantage to aspen, jack pine, black spruce, and birch over white spruce. Many investigators have reported the ubiquity of charcoal under the forest floor and in the upper soil profile.[47] Charcoal in soils provided Bryson et al. (1965) with clues about the forest history of an area 280 km north of the then current tree line at Ennadai Lake, District Keewatin, Northwest Territories.[48]

Two lines of evidence support the thesis that fire has always been an integral factor in the boreal forest: (1) direct, eye-witness accounts and forest-fire statistics, and (2) indirect, circumstantial evidence based on the effects of fire, as well as on persisting indicators.[46] The patchwork mosaic of forest stands in the boreal forest, typically with abrupt, irregular boundaries circumscribing homogenous stands, is indirect but compelling testimony to the role of fire in shaping the forest. The fact is that most boreal forest stands are less than 100 years old, and only in the rather few areas that have escaped burning are there stands of white spruce older than 250 years.[46] The prevalence of fire-adaptive morphologic and reproductive characteristics of many boreal plant species is further evidence pointing to a long and intimate association with fire. Seven of the ten most common trees in the boreal forest—jack pine, lodgepole pine, aspen, balsam poplar (Populus balsamifera), paper birch, tamarack, black spruce – can be classed as pioneers in their adaptations for rapid invasion of open areas. White spruce shows some pioneering abilities, too, but is less able than black spruce and the pines to disperse seed at all seasons. Only balsam fir and alpine fir seem to be poorly adapted to reproduce after fire, as their cones disintegrate at maturity, leaving no seed in the crowns.
The oldest forests in the northwest boreal region, some older than 300 years, are of white spruce occurring as pure stands on moist floodplains.[49] Here, the frequency of fire is much less than on adjacent uplands dominated by pine, black spruce and aspen. In contrast, in the Cordilleran region, fire is most frequent in the valley bottoms, decreasing upward, as shown by a mosaic of young pioneer pine and broadleaf stands below, and older spruce–fir on the slopes above.[46] Without fire, the boreal forest would become more and more homogeneous, with the long-lived white spruce gradually replacing pine, aspen, balsam poplar, and birch, and perhaps even black spruce, except on the peatlands.[50]
Threats
Human activities
Large areas of Siberia's taiga have been harvested for lumber since the collapse of the Soviet Union. Previously, the forest was protected by the restrictions of the Soviet Forest Ministry, but with the collapse of the Union, the restrictions regarding trade with Western nations have vanished. Trees are easy to harvest and sell well, so loggers have begun harvesting Russian taiga evergreen trees for sale to nations previously forbidden by Soviet law.[51]
In Canada, eight percent of the taiga is protected from development, the provincial government allows forest management to occur on Crown land under rigorous constraints.
The main forestry practice in the boreal forest of Canada is clearcutting, which involves cutting down most of the trees in a given area, then replanting the forest as a monocrop (one species of tree) the following season.
Some of the products from logged boreal forests include toilet paper, copy paper, newsprint, and lumber. More than 90% of boreal forest products from Canada are exported for consumption and processing in the United States.
Some of the larger cities situated in this biome are Murmansk,[52] Arkhangelsk, Yakutsk, Anchorage,[53] Yellowknife, Tromsø, Luleå, and Oulu.
Most companies that harvest in Canadian forests are certified by an independent third party agency such as the Forest Stewardship Council (FSC), Sustainable Forests Initiative (SFI), or the Canadian Standards Association (CSA). While the certification process differs between these groups, all of them include forest stewardship, respect for aboriginal peoples, compliance with local, provincial or national environmental laws, forest worker safety, education and training, and other environmental, business, and social requirements. The prompt renewal of all harvest sites by planting or natural renewal is also required.
Climate change
.jpg)
During the last quarter of the twentieth century, the zone of latitude occupied by the boreal forest experienced some of the greatest temperature increases on Earth. Winter temperatures have increased more than summer temperatures. The number of days with extremely cold temperatures (e.g., −20 to −40 °C (−4 to −40 °F) has decreased irregularly but systematically in nearly all the boreal region, allowing better survival for tree-damaging insects.[54] In summer, the daily low temperature has increased more than the daily high temperature.[55] In Fairbanks, Alaska, the length of the frost-free season has increased from 60–90 days in the early twentieth century to about 120 days a century later. Summer warming has been shown to increase water stress and reduce tree growth in dry areas of the southern boreal forest in central Alaska, western Canada and portions of far eastern Russia. Precipitation is relatively abundant in Scandinavia, Finland, northwest Russia and eastern Canada, where a longer growth season (i.e. the period when sap flow is not impeded by frozen water) accelerate tree growth. As a consequence of this warming trend, the warmer parts of the boreal forests are susceptible to replacement by grassland, parkland or temperate forest.[56]
In Siberia, the taiga is converting from predominantly needle-shedding larch trees to evergreen conifers in response to a warming climate. This is likely to further accelerate warming, as the evergreen trees will absorb more of the sun's rays. Given the vast size of the area, such a change has the potential to affect areas well outside of the region.[57] In much of the boreal forest in Alaska, the growth of white spruce trees are stunted by unusually warm summers, while trees on some of the coldest fringes of the forest are experiencing faster growth than previously.[58]
Lack of moisture in the warmer summers are also stressing the birch trees of central Alaska.[59]
Insects
Recent years have seen outbreaks of insect pests in forest-destroying plagues: the spruce-bark beetle (Dendroctonus rufipennis) in Yukon and Alaska;[60] the mountain pine beetle in British Columbia; the aspen-leaf miner; the larch sawfly; the spruce budworm (Choristoneura fumiferana);[61] the spruce coneworm.[62]
Pollution
The effect of sulphur dioxide on woody boreal forest species was investigated by Addison et al. (1984),[63] who exposed plants growing on native soils and tailings to 15.2 μmol/m3 (0.34 ppm) of SO2 on CO2 assimilation rate (NAR). The Canadian maximum acceptable limit for atmospheric SO2 is 0.34 ppm. Fumigation with SO2 significantly reduced NAR in all species and produced visible symptoms of injury in 2–20 days. The decrease in NAR of deciduous species (trembling aspen [Populus tremuloides], willow [Salix], green alder [Alnus viridis], and white birch [Betula papyrifera]) was significantly more rapid than of conifers (white spruce, black spruce [Picea mariana], and jack pine [Pinus banksiana]) or an evergreen angiosperm (Labrador tea) growing on a fertilized Brunisol. These metabolic and visible injury responses seemed to be related to the differences in S uptake owing in part to higher gas exchange rates for deciduous species than for conifers. Conifers growing in oil sands tailings responded to SO2 with a significantly more rapid decrease in NAR compared with those growing in the Brunisol, perhaps because of predisposing toxic material in the tailings. However, sulphur uptake and visible symptom development did not differ between conifers growing on the 2 substrates.
Acidification of precipitation by anthropogenic, acid-forming emissions has been associated with damage to vegetation and reduced forest productivity, but 2-year-old white spruce that were subjected to simulated acid rain (at pH 4.6, 3.6, and 2.6) applied weekly for 7 weeks incurred no statistically significant (P 0.05) reduction in growth during the experiment compared with the background control (pH 5.6) (Abouguendia and Baschak 1987).[64] However, symptoms of injury were observed in all treatments, the number of plants and the number of needles affected increased with increasing rain acidity and with time. Scherbatskoy and Klein (1983)[65] found no significant effect of chlorophyll concentration in white spruce at pH 4.3 and 2.8, but Abouguendia and Baschak (1987)[64] found a significant reduction in white spruce at pH 2.6, while the foliar sulphur content significantly greater at pH 2.6 than any of the other treatments.
Protection
Many nations are taking direct steps to protect the ecology of the taiga by prohibiting logging, mining, oil and gas production, and other forms of development. In February 2010 the Canadian government established protection for 13,000 square kilometres of boreal forest by creating a new 10,700-square-kilometre park reserve in the Mealy Mountains area of eastern Canada and a 3,000-square-kilometre waterway provincial park that follows alongside the Eagle River from headwaters to sea.[66]
Two Canadian provincial governments, Ontario and Quebec, introduced measures in 2008 that would protect at least half of their northern boreal forest.[67][68] Although both provinces admitted it will take years to plan, work with Aboriginal and local communities and ultimately map out precise boundaries of the areas off-limits to development, the measures are expected to create some of the largest protected areas networks in the world once completed. Both announcements came the following year after a letter signed by 1,500 scientists called on political leaders to protect at least half of the boreal forest.[69]
The taiga stores enormous quantities of carbon, more than the world's temperate and tropical forests combined, much of it in wetlands and peatland.[70] In fact, current estimates place boreal forests as storing twice as much carbon per unit area as tropical forests.[71]
Natural disturbance
One of the biggest areas of research and a topic still full of unsolved questions is the recurring disturbance of fire and the role it plays in propagating the lichen woodland.[72] The phenomenon of wildfire by lightning strike is the primary determinant of understory vegetation and because of this, it is considered to be the predominant force behind community and ecosystem properties in the lichen woodland.[73] The significance of fire is clearly evident when one considers that understory vegetation influences tree seedling germination in the short term and decomposition of biomass and nutrient availability in the long term.[73] The recurrent cycle of large, damaging fire occurs approximately every 70 to 100 years.[74] Understanding the dynamics of this ecosystem is entangled with discovering the successional paths that the vegetation exhibits after a fire. Trees, shrubs, and lichens all recover from fire-induced damage through vegetative reproduction as well as invasion by propagules.[75] Seeds that have fallen and become buried provide little help in re-establishment of a species. The reappearance of lichens is reasoned to occur because of varying conditions and light/nutrient availability in each different microstate.[75] Several different studies have been done that have led to the formation of the theory that post-fire development can be propagated by any of four pathways: self replacement, species-dominance relay, species replacement, or gap-phase self replacement.[72] Self replacement is simply the re-establishment of the pre-fire dominant species. Species-dominance relay is a sequential attempt of tree species to establish dominance in the canopy. Species replacement is when fires occur in sufficient frequency to interrupt species dominance relay. Gap-Phase Self-Replacement is the least common and so far has only been documented in Western Canada. It is a self replacement of the surviving species into the canopy gaps after a fire kills another species. The particular pathway taken after a fire disturbance depends on how the landscape is able to support trees as well as fire frequency.[76] Fire frequency has a large role in shaping the original inception of the lower forest line of the lichen woodland taiga.
It has been hypothesized by Serge Payette that the spruce-moss forest ecosystem was changed into the lichen woodland biome due to the initiation of two compounded strong disturbances: large fire and the appearance and attack of the spruce budworm.[77] The spruce budworm is a deadly insect to the spruce populations in the southern regions of the taiga. J.P. Jasinski confirmed this theory five years later stating “Their [lichen woodlands] persistence, along with their previous moss forest histories and current occurrence adjacent to closed moss forests, indicate that they are an alternative stable state to the spruce–moss forests”.[78]
Taiga ecoregions
| Alaska Peninsula montane taiga | United States |
| Central Canadian Shield forests | Canada |
| Cook Inlet taiga | United States |
| Copper Plateau taiga | United States |
| Eastern Canadian forests | Canada |
| Eastern Canadian Shield taiga | Canada |
| Interior Alaska-Yukon lowland taiga | Canada, United States |
| Mid-Continental Canadian forests | Canada |
| Midwestern Canadian Shield forests | Canada |
| Muskwa-Slave Lake forests | Canada |
| Newfoundland Highland forests | Canada |
| Northern Canadian Shield taiga | Canada |
| Northern Cordillera forests | Canada |
| Northwest Territories taiga | Canada |
| South Avalon-Burin oceanic barrens | Canada |
| Northern Lake Superior taiga | United States, Canada |
| Southern Hudson Bay taiga | Canada |
| Yukon Interior dry forests | Canada |
See also
- Birds of North American boreal forests
- Boreal Forest Conservation Framework
- Boreal forest of Canada
- Drunken trees, effect of global warming on the taiga
- Intact forest landscape
- Scandinavian and Russian taiga
- Success of fire suppression in northern forests
- Taiga Rescue Network (TRN)
- Agafia Lykov
References
- "taiga | Definition of taiga in English by Oxford Dictionaries". Oxford Dictionaries | English. Retrieved 2018-07-17.
- "Definition of taiga | Dictionary.com". dictionary.com. Retrieved 2019-06-12.
- "Berkeley: The forest biome". Ucmp.berkeley.edu. Archived from the original on 2019-06-20. Retrieved 2019-05-12.
- "List of Plants & Animals in the Canadian Wilderness". Trails.com. 2010-07-27. Retrieved 2016-12-26.
- "Taiga biological station: FAQ". Wilds.m.ca. Retrieved 2011-02-21.
- "radford:Taiga climate". Radford.edu. Archived from the original on 2011-06-09. Retrieved 2011-02-21.
- Encyclopedia Universalis édition 1976 Vol. 2 ASIE – Géographie physique, p. 568 (in French)
- "Marietta the Taiga and Boreal forest". Marietta.edu. Retrieved 2011-02-21.
- "Yakutsk climate". Worldclimate.com. 2007-02-04. Retrieved 2011-02-21.
- "Interior Alaska-Yukon lowland taiga". Terrestrial Ecoregions. World Wildlife Fund. Retrieved 2011-02-21.
- "The eastern forest – boreal transition". Terrestrial Ecoregions. World Wildlife Fund. Retrieved 2011-02-21.
- Canada: Taiga Shield reference
- "Climate of Canadian ecozones". Geography.ridley.on.ca. Archived from the original on 2011-05-05. Retrieved 2011-02-21.
- "Taiga". Blueplanetbiomes. Archived from the original on 2011-04-10. Retrieved 2011-02-21.
- "Southwest Yukon:Frost-free days". Yukon.taiga.net. Archived from the original on 2011-07-24. Retrieved 2011-02-21.
- "Kenozersky National Park". Wild-russia.org. Retrieved 2011-02-21.
- "University of Helsinki: Carabid diversity in Finnish taiga" (PDF). Retrieved 2011-02-21.
- "Tundra". Blueplanetbiomes. Retrieved 2011-02-21.
- "NatureWorks:Tundra". Nhptv.org. Retrieved 2011-02-21.
- "The Arctic". saskschools.ca. Archived from the original on 2011-04-10. Retrieved 2011-02-21.
- "Finland vegetation zone and freshwater biome" (PDF). 113.139. Retrieved 19 April 2018.
- "Tampere/Pirkkala, Finland Weather History and Climate Data". Worldclimate.com. 2007-02-04. Retrieved 2011-02-21.
- A.P. Sayre, Taiga, (New York: Twenty-First Century Books, 1994) 16.
- Arno & Hammerly 1984, Arno et al. 1995
- Sporrong, Ulf (2003). "The Scandinavian landscape and its resources". In Helle, Knut (ed.). The Cambridge History of Scandinavia. Cambridge University Press. pp. 22.
- Sayre, 19.
- Sayre, 19–20.
- "Study reveals for first time true diversity of life in soils across the globe, new species discovered". Physorg.com. Retrieved 2012-01-14.
- Sayre, 12–13.
- C. Michael Hogan, Black Spruce: Picea mariana, GlobalTwitcher.com, ed. Nicklas Stromberg, November, 2008 Archived 2011-10-05 at the Wayback Machine
- George H. La Roi. "Boreal forest". The Canadian Encyclopedia. Retrieved 2013-11-27.
- "Forest". forest.jrc.ec.europa.eu. Retrieved 2018-02-04.
- Sayre, 23.
- "hww:Nature in the boreal forest biome". Hww.ca. Archived from the original on 2011-01-03. Retrieved 2011-02-21.
- "Wapiti facts and range". Hww.ca. Archived from the original on 2011-01-03. Retrieved 2011-02-21.
- "western roe deer: facts and range". Borealforest.org. Archived from the original on 2011-05-26. Retrieved 2011-02-21.
- "Government of Canada to Send Wood Bison to Russian Conservation Project". Archived from the original on 2013-02-09. Retrieved 2012-12-11.
- "Boreal songbird initiative". Borealbirds.org. Retrieved 2011-02-21.
- Sayre, 28.
- Rowe, J. S. (1955). "Factors influencing white spruce reproduction in Manitoba and Saskatchewan". Can. Dep. Northern Affairs and National Resources. For. Branch, For. Res. Div., Ottawa ON, Project MS-135, Silv. Tech. Note 3.
- Amiro, B. D.; Stocks, B. J.; Alexander, M. E.; Flannigan, M. D.; Wotton, B. M. (2001). "Fire, climate change, carbon and fuel management in the Canadian boreal forest". Internat. J. Wildland Fire. 10 (4): 405–13. doi:10.1071/WF01038.
- Heinselman, M. L. (1981). "Fire intensity and frequency as factors in the distribution and structure of northern ecosystems". Proceedings of the Conference: Fire Regimes in Ecosystem Properties, Dec. 1978, Honolulu, Hawaii. USDA. For. Serv., Washington DC, Gen. Tech. Rep. WO-26. pp. 7–57.
- Heinselman, M. L. (1973). "Fire in the virgin forests of the Boundary Waters Canoe Area, Minnesota". Quat. Res. 3 (3): 329–82. Bibcode:1973QuRes...3..329H. doi:10.1016/0033-5894(73)90003-3.
- Van Wagner, C. E. (1978). "Age-class distribution and the forest cycle". Can. J. For. Res. 8: 220–27. doi:10.1139/x78-034.
- Flannigan, M. D.; Bergeron, Y.; Engelmark, O.; Wotton, B. M. (1998). "Future wildfire in circumboreal forests in relation to global warming". J. Veg. Sci. 9 (4): 469–76. doi:10.2307/3237261. JSTOR 3237261.
- Rowe, J. S.; Scotter, G. W. (1973). "Fire in the boreal forest". Quaternary Res. 3 (3): 444–64. Bibcode:1973QuRes...3..444R. doi:10.1016/0033-5894(73)90008-2. [E3680, Coates et al. 1994]
- La Roi, G. H. (1967). "Ecological studies in the boreal spruce–fir forests of the North American taiga. I. Analysis of the vascular flora". Ecol. Monogr. 37 (3): 229–53. doi:10.2307/1948439. JSTOR 1948439.
- Bryson, R. A.; Irving, W. H.; Larson, J. A. (1965). "Radiocarbon and soil evidence of former forest in the southern Canadian tundra". Science. 147 (3653): 46–48. Bibcode:1965Sci...147...46B. doi:10.1126/science.147.3653.46.
- Rowe, J. S. (1970). "Spruce and fire in northwest Canada and Alaska". In Komarek, E. V. (ed.). Proc. 10th Annual Tall Timbers Fire Ecology Conference, Tallahassee FL. pp. 245–54.
- Raup, H. M.; Denny, C. S. (1950). "Photointerpretation of the terrain along the southern part of the Alaska highway". US Geol. Surv. Bull. 963-D: 95–135. doi:10.3133/b963D.
- "Taiga Deforestation". American.edu. Retrieved 2011-02-21.
- "Murmansk climate". Worldclimate.com. 2007-02-04. Retrieved 2011-02-21.
- "Anchorage climate". Worldclimate.com. 2007-02-04. Retrieved 2011-02-21.
- Seidl, Rupert; Thom, Dominik; Kautz, Markus; Martin-Benito, Dario; Peltoniemi, Mikko; Vacchiano, Giorgio; Wild, Jan; Ascoli, Davide; Petr, Michal; Honkaniemi, Juha; Lexer, Manfred J.; Trotsiuk, Volodymyr; Mairota, Paola; Svoboda, Miroslav; Fabrika, Marek; Nagel, Thomas A.; Reyer, Christopher P. O. (2017-05-31). "Forest disturbances under climate change". Nature. 7 (6): 395–402. Bibcode:2017NatCC...7..395S. doi:10.1038/nclimate3303. PMC 5572641. PMID 28861124.
- Wilmking, M. (2009-10-09). "Coincidence and Contradiction in the Warming Boreal Forest". Geophysical Research Letters. 32 (15). Bibcode:2005GeoRL..3215715W. doi:10.1029/2005GL023331. Retrieved 2012-01-14.
- "Archived copy". Archived from the original on 2011-07-27. Retrieved 2011-03-25.CS1 maint: archived copy as title (link)
- Shuman, Jacquelyn Kremper; Shugart, Herman Henry; O'Halloran, Thomas Liam (2011-03-25). "Russian boreal forests undergoing vegetation change, study shows". Global Change Biology. 17 (7): 2370–84. Bibcode:2011GCBio..17.2370S. doi:10.1111/j.1365-2486.2011.02417.x. Retrieved 2012-01-14.
- "Fairbanks Daily News-Miner – New study states boreal forests shifting as Alaska warms". Newsminer.com. Archived from the original on 2012-01-19. Retrieved 2012-01-14.
- Morello, Lauren. "Forest Changes in Alaska Reveal Changing Climate". Scientific American. Retrieved 2012-01-14.
- "A New Method to Reconstruct Bark Beetle Outbreaks". Colorado.edu. Archived from the original on 2011-06-06. Retrieved 2011-02-21.
- "Spruce budworm and sustainable management of the boreal forest". Cfs.nrcan.gc.ca. 2007-12-05. Archived from the original on 2008-12-02. Retrieved 2011-02-21.
- http://www.fs.fed.us/pnw/pubs/journals/pnw_2006_chapin001.pdf
- Addison, P.A.; Malhotra, S.S.; Khan, A.A. 1984. "Effect of sulfur dioxide on woody boreal forest species grown on native soils and tailings". J. Environ. Qual. 13(3):333–36.
- Abouguendia, Z.M.; Baschak, L.A. 1987. "Response of two western Canadian conifers to simulated acidic precipitation". Water, Air and Soil Pollution 33:15–22.
- Scherbatskoy, T.; Klein, R.M. 1983. "Response of spruce Picea glauca and birch Betula alleghaniensis foliage to leaching by acidic mists". J. Environ. Qual. 12:189–95.
- Braun, David (February 7, 2010). "Boreal landscapes added to Canada's parks Boreal landscapes added to Canada's parks". NatGeo News Watch: News Editor David Braun's Eye on the World. National Geographic Society. Retrieved 17 February 2010.
- Gillespie, Kerry (2008-07-15). "Ontario to protect vast tract". Toronto Star. Retrieved 25 June 2012.
- Marsden, William (2008-11-16). "Charest promises to protect north". Montreal Gazette. Archived from the original on 5 April 2011. Retrieved 25 June 2012.
- "1,500 Scientists Worldwide Call for Protection of Canada's Boreal Forest". Retrieved 25 June 2012.
- Ruckstuhl, K. E.; Johnson, E. A.; Miyanishi, K. (July 2008). "Boreal forest and global change". Philos. Trans. R. Soc. Lond. B Biol. Sci. 363 (1501): 2245–49. doi:10.1098/rstb.2007.2196. PMC 2387060. PMID 18006417.
- "Report: The Carbon the World Forgot". Boreal Songbird Initiative. 2014-05-12.
- Kurkowski, 1911.
- Nilsson, 421.
- Johnson, 212.
- Johnson, 200
- Kurkowski, 1912.
- Payette, 289.
- Jasinski, 561.
- General references
- Arno, S. F. & Hammerly, R. P. (1984). Timberline. Mountain and Arctic Forest Frontiers. Seattle: The Mountaineers. ISBN 0-89886-085-7.
- Arno, S. F.; Worral, J. & Carlson, C. E. (1995). "Larix lyallii: Colonist of tree line and talus sites". In Schmidt, W. C. & McDonald, K. J. (eds.). Ecology and Management of Larix Forests: A Look Ahead. USDA Forest Service General Technical Report GTR-INT-319. pp. 72–78.
- Hoffmann, Robert S. (1958). "The Meaning of the Word 'Taiga'". Ecology. 39 (3): 540–541. doi:10.2307/1931768. JSTOR 1931768.
- Nilsson, M. C. (2005). "Understory vegetation as a forest ecosystem driver, evidence from the northern Swedish boreal forest". Frontiers in Ecology and the Environment. 3 (8): 421–428. doi:10.1890/1540-9295(2005)003[0421:UVAAFE]2.0.CO;2.
- Kurkowski, Thomas (2008). "Relative Importance of Different Secondary Successional Pathways in an Alaskan Boreal Forest". Canadian Journal of Forest Research. 38 (7): 1911–1923. doi:10.1139/X08-039.
- Payette, Serge (2000). "Origin of the lichen woodland at its southern range limit in eastern Canada: the catastrophic impact of insect defoliators and fire on the spruce-moss forest". Canadian Journal of Forest Research. 30 (2): 288–305. doi:10.1139/x99-207.
- Johnson, E. A. (1981). "Vegetation Organization and Dynamics of Lichen Woodland Communities in the Northwest Territories". Ecology. 62 (1): 200–215. doi:10.2307/1936682. JSTOR 1936682.
- Jasinski, J. P. (2005). "The Creation of Alternative Stable States in Southern Boreal Forest: Quebec, Canada". Ecological Monographs. 75 (4): 561–583. doi:10.1890/04-1621.
Further reading
- Sayre, April Pulley (1994), Taiga, Twenty-First Century Books, ISBN 978-0-8050-2830-0
- Gawthrop, Daniel (1999), Vanishing Halo: Saving the Boreal Forest, Greystone Books/David Suzuki Foundation, ISBN 978-0-89886-681-0
- Day, Trevor; Richard Garratt (2006), Taiga, Facts On File, ISBN 978-0-8160-5329-2
External links
| Wikimedia Commons has media related to Taiga. |
- The Conservation Value of the North American Boreal Forest from an Ethnobotanical perspective a report by the Boreal Songbird Initiative
- Boreal Canadian Initiative
- International Boreal Conservation campaign
- Tundra and Taiga
- Threats to Boreal Forests Greenpeace
- Campaign against lumber giant Weyerhaeuser's logging practices in the Canadian boreal forest Rainforest Action Network
- Arctic and Taiga Canadian Geographic
- Terraformers Canadian Taiga Conservation Foundation
- Coniferous Forest, Earth Observatory NASA
- Taiga Rescue Network (TRN) A network of NGOs, indigenous peoples or individuals that works to protect the boreal forests.
- Index of Boreal Forests/Taiga ecoregions at bioimages.vanderbilt.edu
- The Canadian Boreal Forest The Nature Conservancy and its partners
- Slater museum of natural history: Taiga
- Taiga Biological Station founded by Dr. William (Bill) Pruitt, Jr., University of Manitoba.