Finasteride
Finasteride, sold under the brand names Proscar and Propecia, among others, is a medication mainly used to treat an enlarged prostate or hair loss in men.[2] It can also be used to treat excessive hair growth in women and as a part of hormone therapy for transgender women.[3][4] It is taken by mouth.[2]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Proscar, Propecia, others |
| Other names | MK-906; YM-152; L-652,931; 17β-(N-tert-Butylcarbamoyl)-4-aza-5α-androst-1-en-3-one; N-(1,1-Dimethylethyl)-3-oxo-4-aza-5α-androst-1-ene-17β-carboxamide |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a698016 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | 5α-Reductase inhibitor |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 65%[1] |
| Protein binding | 90%[1] |
| Metabolism | Liver (CYP3A4, ALDH)[1] |
| Elimination half-life | Adults: 5–6 hours[1] Elderly: >8 hours[1] |
| Excretion | Feces: 57%[1] Urine: 40%[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.149.445 |
| Chemical and physical data | |
| Formula | C23H36N2O2 |
| Molar mass | 372.553 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Finasteride is a 5α-reductase inhibitor and therefore an antiandrogen.[5] It works by decreasing the production of dihydrotestosterone (DHT) by about 70%, including in the prostate gland and the scalp.[2]
Side effects from finasteride are rare,[6] however some men experience sexual dysfunction, depression, and breast enlargement.[7][8] In some men, sexual dysfunction may persist after stopping the medication.[9][10] It may also increase the risk of certain forms of prostate cancer.[8]
Finasteride was patented in 1984 and approved for medical use in 1992.[11] It is available as a generic medication.[12] A month supply in the United Kingdom costs the NHS about £0.89 per month as of 2019.[13] In the United States, the wholesale cost of this amount is about US$2.34.[14] In 2017, it was the 86th-most commonly prescribed medication in the United States, with more than nine million prescriptions.[15][16]
Medical uses
Enlarged prostate
Physicians sometimes prescribe finasteride for the treatment of benign prostatic hyperplasia (BPH), informally known as an enlarged prostate. Finasteride may improve the symptoms associated with BPH such as difficulty urinating, getting up during the night to urinate, hesitation at the start and end of urination, and decreased urinary flow. It provides less symptomatic relief than alpha-1 blockers such as tamsulosin and symptomatic relief is slower in onset (six months or more of treatment with finasteride may be required to determine the therapeutic results of treatment). Symptomatic benefits are mainly seen in those with prostate volume > 40 cm3. In long-term studies finasteride but not alpha-1 inhibitors reduce the risk of acute urinary retention (−57% at 4 years) and the need for surgery (−54% at 4 years). If the drug is discontinued, any therapeutic benefits are reversed within about 6–8 months.[17][6]
Scalp hair loss
Finasteride is also used to treat male pattern baldness (androgenic alopecia) in men, a condition that develops in up to 80% of Caucasian men.[18][19] In the United States, finasteride and minoxidil are the only two FDA approved drugs for the treatment of male pattern hair loss as of 2017.[20] Treatment with finasteride slows further hair loss[21] and provides about 30% improvement in hair loss after six months of treatment, with effectiveness persisting as long as the drug is taken.[8] Taking finasteride leads to a reduction in scalp and serum DHT levels; by lowering scalp levels of DHT, finasteride can maintain or increase the amount of terminal hairs in the anagen phase by inhibiting and sometimes reversing miniaturization of the hair follicle. Finasteride is most effective on the vertex but can reduce hair loss in all areas of the scalp.[22][23] Finasteride has also been tested for pattern hair loss in women; however, the results were no better than placebo.[24]
Prostate cancer
In males over 55 years old finasteride decreases the risk of low grade prostate cancer but may increase the risk of high grade prostate cancer and has no effect on overall survival.[25]
A 2010 review found a 25% reduction in the risk of prostate cancer with 5α-reductase inhibitor.[26] A follow-up study of the Medicare claims of participants in a 10-year Prostate Cancer Prevention Trial suggests the reduction in prostate cancer is maintained even after discontinuation of treatment.[27] However, 5α-reductase inhibitors have been found to increase the risk of developing certain rare but aggressive forms of prostate cancer (27% risk increase), although not all studies have observed this.[28] No impact of 5-α-reductase inhibitor on survival has been found in people with prostate cancer.[28]
Excessive hair growth
Finasteride has been found to be effective in the treatment of hirsutism (excessive facial and/or body hair growth) in women. In a study of 89 women with hyperandrogenism due to persistent adrenarche syndrome, finasteride produced a 93% reduction in facial hirsutism and a 73% reduction bodily hirsutism after 2 years of treatment. Other studies using finasteride for hirsutism have also found it to be clearly effective.[3]
Transgender hormone therapy
Finasteride is sometimes used in hormone replacement therapy for transgender women due to its antiandrogenic effects, in combination with a form of estrogen. However, little clinical research of finasteride use for this purpose has been conducted and evidence of safety or efficacy is limited.[4] Moreover, caution has been recommended when prescribing finasteride to transgender women, as finasteride may be associated with side effects such as depression, anxiety, and suicidal ideation, symptoms that are particularly prevalent in the transgender population and in others at high risk already.[29]
Adverse effects
A 2010 Cochrane review concluded that adverse effects from finasteride are rare when used for BPH.[6] When finasteride was originally approved for hair loss in 1997, the FDA reported that it appeared well tolerated, with the most common side effects being related to sexual function.[30] Finasteride is contraindicated in pregnancy.[31][32] The Food and Drug Administration advises that donation of blood or plasma be deferred for at least one month after taking the last dose of finasteride.[33]
The FDA has added a warning to 5α-reductase inhibitors concerning an increased risk of high-grade prostate cancer, as the treatment of BPH lowers PSA (prostate-specific antigen), which could mask the development of prostate cancer.[34][35] Although overall incidence of male breast cancer in clinical trials for finasteride 5 mg was not increased, there are post-marketing reports of breast cancer in association with its use, though available evidence does not provide clarity as to whether there is a causative relationship between finasteride and these cancers.[19][36] A 2018 meta-analysis found no higher risk of breast cancer with 5α-reductase inhibitors.[37] Some men develop gynecomastia (breast development or enlargement) following finasteride usage.[38][39][40][41] The risk of gynecomastia with 5α-reductase inhibitors is low at about 1.5%.[42] Depressive symptoms and suicidality have been reported.[43]
Sexual dysfunction
Finasteride causes short-term sexual dysfunction in some men, which may persist in some men after stopping the medication.[9][10] There are case reports of persistent diminished libido or erectile dysfunction after stopping the drug and the FDA has updated the label to inform people of these reports.[8][44][42]
The 2010 Cochrane review found that compared with placebo, men taking finasteride are at increased risk for impotence, erectile dysfunction, decreased libido, and ejaculation disorder for the first year of treatment. The risk of side effects were dose dependent, with a higher risk for men taking 5 mg in comparison to 1 mg.[45] The rates also became indistinguishable from placebo after 2–4 years and these side effects usually got better over time.[6][46] A 2016 meta-analysis found that sexual dysfunction, including erectile dysfunction, loss of libido, and reduced ejaculate, may occur in 3.4 to 15.8% of men treated with finasteride or dutasteride.[28] This adverse effect has been linked to lower quality of life and can cause stress in relationships.[47]
Long-term
Finasteride may cause persistent adverse sexual, neurological and physical effects in a subset of men.[9][10] This has been called post-finasteride syndrome, characterized by reported sexual and physical symptoms such as loss of libidio, erectile dysfunction, ejaculatory disorders, reduction in penis size, penile curvature, reduced sensation, male breast enlargement, muscular atrophy, fatigue and severely dry skin. People with post-finasteride syndrome may experience depression and anxiety, cognitive impairment and suicidal thoughts.[10]
Post-finasteride syndrome may also have reduced levels of neurosteroids such as allopregnanolone in their cerebrospinal fluid. One study found that 1.4% developed persistent sexual dysfunction.[9]
A 2019 Reuters investigation showed that Merck found evidence of persistent side effects in their original clinical trials and did not disclose it in their warning label.[48][49] They uncovered court filings which demonstrated that Merck misrepresented Propecia's safety record following clinical trials in the mid-late 1990s.[48]
In one deposition, Charlotte Merritt, who oversaw regulatory activity for Propecia, acknowledged that Merck changed Propecia's label for sexual adverse events in 2002, four years after Propecia hit the market. The label changed from “resolution occurred in all men who discontinued therapy with Propecia ” to “resolution occurred in men who discontinued therapy with Propecia”. Merritt testified that Merck eliminated the word “all” due to evidence from the clinical trials of adverse events that did not resolve following discontinuation of use.[49] In another deposition, Paul Howes, the head of marketing for Propecia acknowledged that Merck was aware that warnings of sexual side effects, particularly persistent to permanent side effects, would have a devastating impact on sales.[49]
Overdose
Finasteride has been studied in humans at single doses of up to 400 mg and at continuous dosages of up to 80 mg/day for three months, without adverse effects observed.[19][17][50] These doses are many times in excess of the typical dosages of finasteride of 1 mg/day and 5 mg/day that are used clinically.[19][17] There is no specific recommended antidote for finasteride overdose.[19][17]
Interactions
No significant drug interactions have been observed between finasteride and a limited selection of medications.[51]
Pharmacology
Pharmacodynamics
Finasteride is a 5α-reductase inhibitor.[19][1] It is specifically a selective inhibitor of the type II and III isoforms of the enzyme.[1][52][53] By inhibiting these two isozymes of 5α-reductase, finasteride reduces the formation of the potent androgen dihydrotestosterone (DHT) from its precursor testosterone in certain tissues in the body such as the prostate gland, skin, and hair follicles.[1][54] As such, finasteride is a type of antiandrogen, or more specifically, an androgen synthesis inhibitor.[55][56] However, some authors do not define finasteride as an "antiandrogen," a term which can refer more specifically to antagonists of the androgen receptor.[57]
Finasteride results in a decrease of circulating DHT levels by about 65 to 70% with an oral dosage of 5 mg/day and of DHT levels in the prostate gland by up to 80 to 90% with an oral dosage of 1 or 5 mg/day.[52][58][59] In parallel, circulating levels of testosterone increase by approximately 10%, while local concentrations of testosterone in the prostate gland increase by about 7-fold and local testosterone levels in hair follicles increase by around 27 to 53%.[60][61] An oral dosage of finasteride of only 0.2 mg/day has been found to achieve near-maximal suppression of DHT levels (68.6% for 0.2 mg/day relative to 72.2% for 5 mg/day).[61][62] Finasteride does not completely suppress DHT production because it lacks significant inhibitory effects on the 5α-reductase type I isoenzyme, with more than 100-fold less inhibitory potency for type I as compared to type II (IC50 = 313 nM and 11 nM, respectively).[19][1] This is in contrast to inhibitors of all three isoenzymes of 5α-reductase like dutasteride, which can reduce DHT levels in the entire body by more than 99%.[52] In addition to inhibiting 5α-reductase, finasteride has also been found to competitively inhibit 5β-reductase (AKR1D1).[63] However, its affinity for the enzyme is substantially less than for 5α-reductase (an order of magnitude less than for 5α-reductase type I) and hence is unlikely to be of clinical significance.[63]
As of 2012, the tissues in which the different isozymes of 5α-reductase are expressed are not fully clear.[54] This is because different investigators have obtained varying results with different reagents, methods, and tissues examined.[54] However, the different isozymes of 5α-reductase appear to be widely expressed, with notable tissues including the prostate gland, seminal vesicles, testes, epididymides, skin, hair follicles, liver, kidneys, and brain, among others.[54]
By inhibiting 5α-reductase and thus preventing DHT production, finasteride reduces androgen signaling in tissues like the prostate gland and the scalp. In the prostate, this reduces prostate volume, which improves BPH and reduces risk of prostate cancer. Finasteride reduces prostate volume by 20 to 30% in men with benign prostatic hyperplasia.[64] Inhibition of 5α-reductase also reduces epididymal weight, and decreases motility and normal morphology of spermatozoa in the epididymis.[65]
Neurosteroids like 3α-androstanediol (derived from DHT) and allopregnanolone (derived from progesterone) activate the GABAA receptor in the brain; because finasteride prevents the formation of neurosteroids, it functions as a neurosteroidogenesis inhibitor and may contribute to a reduction of GABAA activity. Reduction of GABAA receptor activation by these neurosteroids has been implicated in depression, anxiety, and sexual dysfunction.[66][67][68]
Pharmacokinetics
The mean oral bioavailability of finasteride is approximately 65%.[1] The absorption of finasteride is not affected by food.[19][17] At steady-state with 1 mg/day finasteride, mean peak concentrations of finasteride were 9.2 ng/mL (25 nmol/L).[19] Conversely, following a single 5 mg dose of finasteride, mean peak levels of finasteride were 37 ng/mL (99 nmol/L), and plasma concentrations increased by 47 to 54% following 2.5 weeks of continued daily administration.[17] The volume of distribution of finasteride is 76 L.[1] Its plasma protein binding is 90%.[1] The drug has been found to cross the blood–brain barrier, whereas levels in semen were found to be undetectable.[1]
Finasteride is extensively metabolized in the liver, first by hydroxylation via CYP3A4 and then by aldehyde dehydrogenase.[1] It has two major metabolites, which are the tert-butyl side chain monohydroxylated and monocarboxylic acid metabolites.[1] These metabolites show approximately 20% of the inhibitory activity of finasteride on 5α-reductase.[1] Hence, the metabolites of finasteride are not particularly active.[1] The drug has a terminal half-life of 5 to 6 hours in adult men (18–60 years of age) and a terminal half-life of 8 hours or more in elderly men (more than 70 years of age).[1] It is eliminated as its metabolites 57% in the feces and 40% in the urine.[1]
Chemistry
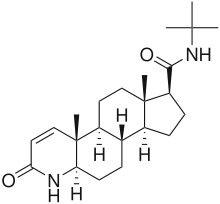
Finasteride, also known as 17β-(N-tert-butylcarbamoyl)-4-aza-5α-androst-1-en-3-one, is a synthetic androstane steroid and 4-azasteroid.[51][69] It is an analogue of androgen steroid hormones like testosterone and DHT.[51] As an unconjugated steroid, finasteride is a highly lipophilic compound.[51][70]
History
In 1942, James Hamilton observed that prepubertal castration prevents the later development of male pattern baldness in mature men.[71] In 1974, Julianne Imperato-McGinley of Cornell Medical College in New York attended a conference on birth defects. She reported on a group of intersex children in the Caribbean who appeared sexually ambiguous at birth, and were initially raised as girls, but then grew external male genitalia and other masculine characteristic after onset of puberty. These children, despite being raised as girls until puberty, were generally heterosexual, and were termed "Guevedoces" by their local community, which means "penis at twelve" in Spanish.[72] Her research group found these children shared a genetic mutation, causing deficiency of the 5α-reductase enzyme and male hormone dihydrotestosterone (DHT), which was found to have been the etiology behind abnormalities in male sexual development. Upon maturation, these individuals were observed to have smaller prostates which were underdeveloped, and were also observed to lack incidence of male pattern baldness.[73][74]
In 1975, copies of Imperato-McGinley's presentation were seen by P. Roy Vagelos, who was then serving as Merck's basic-research chief. He was intrigued by the notion that decreased levels of DHT led to the development of smaller prostates. Dr. Vagelos then sought to create a drug which could mimic the condition found in these children to treat older men who were suffering from benign prostatic hyperplasia.[75]
Finasteride was developed by Merck under the code name MK-906.[51] A team led by chemist Gary Rasmusson and biologist Jerry Brooks developed potential 5α-reductase inhibitors based on transition state inhibitors, using an iterative process of molecular design, testing, and redesign.[76] In 1992, finasteride (5 mg) was approved by the U.S. Food and Drug Administration (FDA) for treatment of BPH, which Merck marketed under the brand name Proscar. Rasmusson and Brooks were awarded IPO's “Inventor of the Year” award in 1993 for their work on finasteride.[77] In 1997, Merck was successful in obtaining FDA approval for a second indication of finasteride (1 mg) for treatment of male pattern hair loss, which was marketed under the brand name Propecia.[78] It was the first 5α-reductase inhibitor to be introduced and was followed by dutasteride in 2001.[79] The first study of finasteride in the treatment of hirsutism in women was published in 1994.[80]
Society and culture
Generic names
Finasteride is the generic name of the drug and its INN, USAN, BAN, and JAN, while finastéride is its DCF.[81][82][83][84] It is also known by its former developmental code names MK-906, YM-152, and L-652,931.[81][82][83][84]
Brand names
Finasteride is marketed primarily under the brand names Propecia, for pattern hair loss, and Proscar, for BPH, both of which are products of Merck & Co.[84] There is 1 mg of finasteride in Propecia and 5 mg in Proscar. Merck's patent on finasteride for the treatment of BPH expired in June 2006.[85] Merck was awarded a separate patent for the use of finasteride to treat pattern hair loss and it expired in November 2013.[86] Finasteride is also marketed under a variety of other brand names throughout the world.[84]
Controversy
Men in the U.S. and Canada concerned about persistent sexual side effects "coined the phrase 'post-finasteride syndrome', which they say is characterized by sexual, neurological, hormonal and psychological side effects that can persist in men who have taken finasteride for hair loss or an enlarged prostate".[87] In 2016, Merck was a defendant in approximately 1,370 product liability lawsuits which had been filed by customers alleging they have experienced persistent sexual side effects following cessation of treatment with finasteride.[88]
Athletics
From 2005 to 2009, the World Anti-Doping Agency banned finasteride because it was discovered that the drug could be used to mask steroid abuse.[89] It was removed from the list effective January 1, 2009, after improvements in testing methods made the ban unnecessary.[90] Athletes who used finasteride and were banned from international competition include skeleton racer Zach Lund, bobsledder Sebastien Gattuso, footballer Romário, and ice hockey goaltender José Théodore.[90][91]
Miscellaneous
The U.S. Food and Drug Administration advises that donation of blood or plasma be deferred for at least one month after taking the last dose of finasteride.[92] The UK also has a one-month deferral period.[93]
Harold Bornstein, the former[94] personal physician of United States President Donald Trump, stated in 2017 that Trump was taking finasteride to promote hair growth.[95][96]
Research
Preliminary research suggests that topical finasteride may be effective in the treatment of pattern hair loss.[97][98] Topical finasteride, like the oral preparation, reduces serum DHT.[98] However, studies have shown that the extent of the reduction of serum DHT is less with the topical application.
DHT may be involved in the cause of acne, and 5α-reductase inhibitors might be effective in the treatment of the condition.[99][100] A small retrospective study reported that finasteride was effective in the treatment of acne in women with normal testosterone levels.[101][100] A randomized controlled trial found that finasteride was less effective than flutamide or an ethinylestradiol/cyproterone acetate birth control pill in the treatment of acne in women with high androgen levels.[101]
Androgens and estrogens may be involved in the cause of hidradenitis suppurativa (acne inversa).[102][103] Two case series have reported that finasteride is effective in the treatment of hidradenitis suppurativa in girls and women.[101]
References
- Lemke TL, Williams DA (2008). Foye's Principles of Medicinal Chemistry (6th ed.). Lippincott Williams & Wilkins. pp. 1286–. ISBN 978-0-7817-6879-5.
- "Finasteride Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Retrieved 5 March 2019.
- Blume-Peytavi U, Whiting DA, Trüeb RM (26 June 2008). Hair Growth and Disorders. Springer Science & Business Media. p. 369. ISBN 978-3-540-46911-7.
- Knezevich EL, Viereck LK, Drincic AT (January 2012). "Medical management of adult transsexual persons". Pharmacotherapy. 32 (1): 54–66. doi:10.1002/PHAR.1006. PMID 22392828.
- Ferri, Fred F. (2014). Ferri's Clinical Advisor 2015 E-Book: 5 Books in 1. Elsevier Health Sciences. p. 580. ISBN 9780323084307.
- Tacklind J, Fink HA, Macdonald R, Rutks I, Wilt TJ (October 2010). "Finasteride for benign prostatic hyperplasia". The Cochrane Database of Systematic Reviews (10): CD006015. doi:10.1002/14651858.CD006015.pub3. PMID 20927745.
- Zakhem GA, Goldberg JE, Motosko CC, Cohen BE, Ho RS (1 July 2019). "Sexual dysfunction in men taking systemic dermatologic medication: A systematic review". J Am Acad Dermatol. 81 (1): 163–172. doi:10.1016/j.jaad.2019.03.043. PMID 30905792.
- Varothai S, Bergfeld WF (July 2014). "Androgenetic alopecia: an evidence-based treatment update". American Journal of Clinical Dermatology. 15 (3): 217–30. doi:10.1007/s40257-014-0077-5. PMID 24848508.
- Zakhem, GA; Goldberg, JE; Motosko, CC; Cohen, BE; Ho, RS (July 2019). "Sexual dysfunction in men taking systemic dermatologic medication: A systematic review". Journal of the American Academy of Dermatology. 81 (1): 163–172. doi:10.1016/j.jaad.2019.03.043. PMID 30905792.
In studies addressing reversibility, most of these patients have resolution of sexual adverse effects after discontinuation of finasteride, and many have improvement of adverse effects over time with continued finasteride use. However, some studies CAPSULE SUMMARY d Prescription medications are a common cause of sexual dysfunction. Possible sexual adverse effects should be discussed with men using these medications. d We identified evidence for sexual adverse effects in patients taking 11 systemic dermatologic medications. Level 1 evidence evaluating sexual dysfunction as a primary outcome was available for finasteride.
- Traish, AM (January 2020). "Post-finasteride syndrome: a surmountable challenge for clinicians". Fertility and Sterility. 113 (1): 21–50. doi:10.1016/j.fertnstert.2019.11.030. PMID 32033719.
- Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 483. ISBN 9783527607495.
- Sataloff RT, Sclafani AP (30 November 2015). Sataloff's Comprehensive Textbook of Otolaryngology: Head & Neck Surgery: Facial Plastic and Reconstructive Surgery. JP Medical Ltd. pp. 400–. ISBN 978-93-5152-459-5.
- British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. p. 769. ISBN 9780857113382.
- "NADAC as of 2019-02-27". Centers for Medicare and Medicaid Services. Retrieved 3 March 2019.
- "The Top 300 of 2020". ClinCalc. Retrieved 11 April 2020.
- "Finasteride - Drug Usage Statistics". ClinCalc. Retrieved 11 April 2020.
- Proscar label
- Kanti V1, Messenger A2, Dobos G1, Reygagne P3, Finner A4, Blumeyer A5, Trakatelli M6, Tosti A7,8, Del Marmol V9, Piraccini BM10, Nast A11, Blume-Peytavi U1. Evidence-based (S3) guideline for the treatment of androgenetic alopecia in women and in men - short version. J Eur Acad Dermatol Venereol. 2018 Jan;32(1):11-22. doi: 10.1111/jdv.14624. Epub 2017 Nov 27.
- "Propecia label" (PDF).
- Adil A, Godwin M (July 2017). "The effectiveness of treatments for androgenetic alopecia: A systematic review and meta-analysis". Journal of the American Academy of Dermatology. 77 (1): 136–141.e5. doi:10.1016/j.jaad.2017.02.054. PMID 28396101.
- Habif TP (23 April 2015). Clinical Dermatology. Elsevier Health Sciences. pp. 934–. ISBN 978-0-323-26607-9.
- Yim E, Nole KL, Tosti A (December 2014). "5α-Reductase inhibitors in androgenetic alopecia". Current Opinion in Endocrinology, Diabetes and Obesity. 21 (6): 493–8. doi:10.1097/MED.0000000000000112. PMID 25268732.
- Gupta AK, Charrette A (April 2014). "The efficacy and safety of 5α-reductase inhibitors in androgenetic alopecia: a network meta-analysis and benefit-risk assessment of finasteride and dutasteride". The Journal of Dermatological Treatment. 25 (2): 156–61. doi:10.3109/09546634.2013.813011. PMID 23768246.
- Levy LL, Emer JJ (August 2013). "Female pattern alopecia: current perspectives". International Journal of Women's Health. 5: 541–56. doi:10.2147/IJWH.S49337. PMC 3769411. PMID 24039457.
- "Finasteride for Prostate Cancer Prevention". National Cancer Institute. 28 August 2013. Retrieved 8 February 2020.
- Wilt TJ, Macdonald R, Hagerty K, Schellhammer P, Tacklind J, Somerfield MR, Kramer BS (2010). "5-α-Reductase inhibitors for prostate cancer chemoprevention: an updated Cochrane systematic review". BJU Int. 106 (10): 1444–51. doi:10.1111/j.1464-410X.2010.09714.x. PMID 20977593.
- Unger JM, Hershman DL, Till C, Tangen CM, Barlow WE, Ramsey SD, Goodman PJ, Thompson IM (March 2018). "Using Medicare Claims to Examine Long-term Prostate Cancer Risk of Finasteride in the Prostate Cancer Prevention Trial". Journal of the National Cancer Institute. 110 (11): 1208–1215. doi:10.1093/jnci/djy035. PMC 6235685. PMID 29534197.
- Hirshburg JM, Kelsey PA, Therrien CA, Gavino AC, Reichenberg JS (2016). "Adverse Effects and Safety of 5-alpha Reductase Inhibitors (Finasteride, Dutasteride): A Systematic Review". J Clin Aesthet Dermatol. 9 (7): 56–62. PMC 5023004. PMID 27672412.
- Trüeb, Ralph M. (2017). "Discriminating in favour of or against men with increased risk of finasteride-related side effects?". Experimental Dermatology. 26 (6): 527–528. doi:10.1111/exd.13155. ISSN 0906-6705. PMID 27489125.
[...] caution is recommended while prescribing oral finasteride to male-to-female transsexuals, as the drug has been associated with inducing depression, anxiety and suicidal ideation, symptoms that are particularly common in patients with gender dysphoria, who are already at a high risk.[9]
- FDA. "Summary of Key Safety Findings" (PDF). p. 98.
- "PROPECIA Prescribing Information" (PDF). US Food & Drug Administration / Merck & Co., Inc. Retrieved 30 January 2020.
- "PROSCAR Prescribing Information" (PDF). US Food & Drug Administration / Merck & Co., Inc. Retrieved 30 January 2020.
- "Deferral of Blood and Plasma donors – Medications". FDA. 28 July 1993. Retrieved 30 January 2020.
- FDA. Posted 9 June 2011. 5-alpha reductase inhibitors (5-ARIs): Label Change – Increased Risk of Prostate Cancer
- Walsh PC (April 2010). "Chemoprevention of prostate cancer". The New England Journal of Medicine. 362 (13): 1237–8. doi:10.1056/NEJMe1001045. PMID 20357287.
- Medicines and Healthcare products Regulatory Agency Drug Safety Update. December 2009 Finasteride: potential risk of male breast cancer
- Wang J, Zhao S, Luo L, Li E, Li X, Zhao Z (2018). "5-alpha Reductase Inhibitors and risk of male breast cancer: a systematic review and meta-analysis". Int Braz J Urol. 44 (5): 865–873. doi:10.1590/S1677-5538.IBJU.2017.0531. PMC 6237523. PMID 29697934.
- Narula HS, Carlson HE (August 2014). "Gynaecomastia-pathophysiology, diagnosis and treatment". Nat Rev Endocrinol. 10 (11): 684–698. doi:10.1038/nrendo.2014.139. PMID 25112235.
- Deepinder F, Braunstein GD (2012). "Drug-induced gynecomastia: an evidence-based review". Expert Opinion on Drug Safety. 11 (5): 779–795. doi:10.1517/14740338.2012.712109. PMID 22862307.
- Bolignano D, Palmer SC, Navaneethan SD, Strippoli GF (April 2014). "Aldosterone antagonists for preventing the progression of chronic kidney disease". Cochrane Database of Systematic Reviews. 4 (4): CD007004. doi:10.1002/14651858.CD007004.pub3. PMID 24782282.
- Aiman U, Haseeen MA, Rahman SZ (December 2009). "Gynecomastia: An ADR due to drug interaction". Indian Journal of Pharmacology. 41 (6): 286–7. doi:10.4103/0253-7613.59929. PMC 2846505. PMID 20407562.
- Trost L, Saitz TR, Hellstrom WJ (2013). "Side Effects of 5-Alpha Reductase Inhibitors: A Comprehensive Review". Sex Med Rev. 1 (1): 24–41. doi:10.1002/smrj.3. PMID 27784557.
- Locci A, Pinna G (2017). "Neurosteroid biosynthesis downregulation and changes in GABAA receptor subunit composition: A biomarker axis in stress-induced cognitive and emotional impairment". Br. J. Pharmacol. 174 (19): 3226–3241. doi:10.1111/bph.13843. PMC 5595768. PMID 28456011.
- FDA (11 April 2012). "Questions and Answers: Finasteride Label Changes". US FDA. Retrieved 26 October 2014.
- "Persistent erectile dysfunction in men exposed to the 5a-reductase inhibitors, finasteride, or dutasteride" (PDF). 9 March 2017.
- Mella JM, Perret MC, Manzotti M, Catalano HN, Guyatt G (October 2010). "Efficacy and safety of finasteride therapy for androgenetic alopecia: a systematic review". Archives of Dermatology. 146 (10): 1141–50. doi:10.1001/archdermatol.2010.256. PMID 20956649.
- Gur S, Kadowitz PJ, Hellstrom WJ (January 2013). "Effects of 5-alpha reductase inhibitors on erectile function, sexual desire and ejaculation". Expert Opinion on Drug Safety. 12 (1): 81–90. doi:10.1517/14740338.2013.742885. PMID 23173718.
- "U.S. court let Merck hide secrets about popular drug's risks". Reuters. Retrieved 17 February 2020.
- "In re: Propecia (Finasteride) Products" (PDF). Retrieved 17 February 2020.
- Frye SV (2006). "Discovery and clinical development of dutasteride, a potent dual 5alpha-reductase inhibitor". Curr Top Med Chem. 6 (5): 405–21. doi:10.2174/156802606776743101. PMID 16719800.
- Sudduth SL, Koronkowski MJ (1993). "Finasteride: the first 5α-reductase inhibitor". Pharmacotherapy. 13 (4): 309–25, discussion 325–9. doi:10.1002/j.1875-9114.1993.tb02739.x (inactive 7 May 2020). PMID 7689728.
- Yamana K, Labrie F, Luu-The V (August 2010). "Human type 3 5α-reductase is expressed in peripheral tissues at higher levels than types 1 and 2 and its activity is potently inhibited by finasteride and dutasteride". Hormone Molecular Biology and Clinical Investigation. 2 (3): 293–9. doi:10.1515/hmbci.2010.035. PMID 25961201.
- Aggarwal S, Thareja S, Verma A, Bhardwaj TR, Kumar M (February 2010). "An overview on 5alpha-reductase inhibitors". Steroids. 75 (2): 109–53. doi:10.1016/j.steroids.2009.10.005. PMID 19879888.
- Azzouni F, Godoy A, Li Y, Mohler J (2012). "The 5 alpha-reductase isozyme family: a review of basic biology and their role in human diseases". Adv Urol. 2012: 1–18. doi:10.1155/2012/530121. PMC 3253436. PMID 22235201.
- Victor R. Preedy (2012). Handbook of Hair in Health and Disease. Springer Science & Business Media. pp. 89–. ISBN 978-90-8686-728-8.
- Jashin J. Wu (18 October 2012). Comprehensive Dermatologic Drug Therapy E-Book. Elsevier Health Sciences. pp. 361–. ISBN 978-1-4557-3801-4.
- Clapauch, Ruth; Weiss, Rita Vasconcellos; Rech, Ciciliana Maila Zilio (2017). "Testosterone and Women". Testosterone. pp. 319–351. doi:10.1007/978-3-319-46086-4_17. ISBN 978-3-319-46084-0.
Finasteride is not actually an antiandrogen but a 5α-reductase inhibitor.
- Bartsch G, Rittmaster RS, Klocker H (April 2000). "Dihydrotestosterone and the concept of 5alpha-reductase inhibition in human benign prostatic hyperplasia". European Urology. 37 (4): 367–80. doi:10.1159/000020181. PMID 10765065.
- Kim EH, Brockman JA, Andriole GL (January 2018). "The use of 5-alpha reductase inhibitors in the treatment of benign prostatic hyperplasia". Asian Journal of Urology. 5 (1): 28–32. doi:10.1016/j.ajur.2017.11.005. PMC 5780290. PMID 29379733.
- Rittmaster RS (January 1994). "Finasteride". N. Engl. J. Med. 330 (2): 120–5. doi:10.1056/NEJM199401133300208. PMID 7505051.
- Libecco JF, Bergfeld WF (April 2004). "Finasteride in the treatment of alopecia". Expert Opin Pharmacother. 5 (4): 933–40. doi:10.1517/14656566.5.4.933. PMID 15102575.
- Shapiro J, Kaufman KD (June 2003). "Use of finasteride in the treatment of men with androgenetic alopecia (male pattern hair loss)". J. Investig. Dermatol. Symp. Proc. 8 (1): 20–3. doi:10.1046/j.1523-1747.2003.12167.x. PMID 12894990.
- Drury JE, Di Costanzo L, Penning TM, Christianson DW (July 2009). "Inhibition of human steroid 5beta-reductase (AKR1D1) by finasteride and structure of the enzyme-inhibitor complex". The Journal of Biological Chemistry. 284 (30): 19786–90. doi:10.1074/jbc.C109.016931. PMC 2740403. PMID 19515843.
- Bostwick DG, Cheng L (24 January 2014). Urologic Surgical Pathology E-Book. Elsevier Health Sciences. pp. 402–. ISBN 978-0-323-08619-6.
- Robaire B, Henderson NA (May 2006). "Actions of 5alpha-reductase inhibitors on the epididymis". Molecular and Cellular Endocrinology. 250 (1–2): 190–5. doi:10.1016/j.mce.2005.12.044. PMID 16476520.
- Finn DA, Beadles-Bohling AS, Beckley EH, Ford MM, Gililland KR, Gorin-Meyer RE, Wiren KM (2006). "A new look at the 5alpha-reductase inhibitor finasteride". CNS Drug Reviews. 12 (1): 53–76. doi:10.1111/j.1527-3458.2006.00053.x. PMC 6741762. PMID 16834758.
- Römer B, Gass P (December 2010). "Finasteride-induced depression: new insights into possible pathomechanisms". Journal of Cosmetic Dermatology. 9 (4): 331–2. doi:10.1111/j.1473-2165.2010.00533.x. PMID 21122055.
- Gunn BG, Brown AR, Lambert JJ, Belelli D (2011). "Neurosteroids and GABA(A) Receptor Interactions: A Focus on Stress". Frontiers in Neuroscience. 5: 131. doi:10.3389/fnins.2011.00131. PMC 3230140. PMID 22164129.
- Tian G, Stuart JD, Moss ML, Domanico PL, Bramson HN, Patel IR, Kadwell SH, Overton LK, Kost TA, Mook RA (1994). "17 beta-(N-tert-butylcarbamoyl)-4-aza-5 alpha-androstan-1-en-3-one is an active site-directed slow time-dependent inhibitor of human steroid 5 alpha-reductase 1". Biochemistry. 33 (8): 2291–6. doi:10.1021/bi00174a041. PMID 8117686.
- Azeem A, Khan ZI, Aqil M, Ahmad FJ, Khar RK, Talegaonkar S (May 2009). "Microemulsions as a surrogate carrier for dermal drug delivery". Drug Development and Industrial Pharmacy. 35 (5): 525–47. doi:10.1080/03639040802448646. PMID 19016057.
- Hamilton, J (1942). "Male hormone stimulation is prerequisite and an incitant in common baldness". American Journal of Anatomy. 71 (3): 451–480. doi:10.1002/aja.1000710306.
- "The extraordinary case of the Guevedoces". BBC News. 20 September 2015. Retrieved 3 September 2018.
- Imperato-McGinley J, Guerrero L, Gautier T, Peterson RE (December 1974). "Steroid 5alpha-reductase deficiency in man: an inherited form of male pseudohermaphroditism". Science. 186 (4170): 1213–5. Bibcode:1974Sci...186.1213I. doi:10.1126/science.186.4170.1213. PMID 4432067.
- Isfort AH, Emerick JE, Paz RA (11 November 2016). "5-Alpha-Reductase Deficiency". WebMD. News & Perspective Drugs & Diseases CME & Education Academy Consult, Drugs & Diseases > Pediatrics: General Medicine.
- Freudenheim M (16 February 1992). "Keeping the Pipeline Filled at Merck". The New York Times.
- Cordes E (2014). Hallelujah Moments: Tales of Drug Discovery. Oxford University Press. ISBN 9780199337149.
- "Past Inventor of the Year Award Winners". ipoef.org. Intellectual Property Owners Education Foundation. Retrieved 21 June 2020.
- Burger A, Abraham DJ (20 February 2003). Burger's Medicinal Chemistry and Drug Discovery, Autocoids, Diagnostics, and Drugs from New Biology. Wiley. p. 439. ISBN 978-0-471-37030-7.
- Doherty AM (2003). Annual Reports in Medicinal Chemistry. Academic Press. pp. 353–. ISBN 978-0-12-040538-1.
- Diamanti-Kandarakis E, Tolis G, Duleba AJ (1995). "Androgens and therapeutic aspects of antiandrogens in women". J. Soc. Gynecol. Investig. 2 (4): 577–92. doi:10.1177/107155769500200401. PMID 9420861.
- Index Nominum 2000: International Drug Directory. Taylor & Francis. 2000. p. 443. ISBN 978-3-88763-075-1.
- I.K. Morton; Judith M. Hall (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 121–. ISBN 978-94-011-4439-1.
- Barrie W. Bycroft; David J. Payne (9 August 2013). Dictionary of Antibiotics and Related Substances: with CD-ROM, Second Edition. CRC Press. pp. 816–. ISBN 978-1-4822-8215-3.
- "Finasteride".
- "Primary Patent Expirations for Selected High Revenue Drugs". RxNews. Prescription Solutions. Archived from the original on 21 March 2008.
- FDA. "Patent Expiration for Propecia". Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations.
- Margo J (26 September 2012). "Looking at care with a critical eye". Australian Financial Review. Archived from the original on 14 November 2012.
- Daniel Marchalik (4 February 2017). "Watch for these potential side effects in drug Trump reportedly takes for hair loss". Miami Herald. Retrieved 9 December 2018.
- Sandomir R (19 January 2006). "Skin Deep; Fighting Baldness, and Now an Olympic Ban". The New York Times. Retrieved 2 May 2010.
- Staff (28 October 2008). "WADA removes Finasteride from ban list". The Australian.
- Staff (9 October 2008). "WADA takes Romario's drug off banned list". Sydney Morning Herald.
- "Deferral of Blood and Plasma donors – Medications" (PDF). FDA. 28 July 1993. Retrieved 4 February 2017.
- "Anti-Androgens - Joint United Kingdom Blood Transfusion and Tissue Transplantation Services Professional Advisory Committee". www.transfusionguidelines.org. 1 June 2007. Retrieved 13 May 2020.
- Schechter, Anna R. (1 May 2018). "Trump doctor Harold Bornstein says bodyguard, lawyer 'raided' his office, took medical files". NBC News. Retrieved 27 October 2018.
- Altman LK (1 February 2017). "Donald Trump's Longtime Doctor Says President Takes Hair-Growth Drug". The New York Times. Retrieved 25 February 2017.
- "Trump doctor reveals secret to US president's hair". the Guardian. 2 February 2017. Retrieved 13 May 2020.
- Lee SW, Juhasz M, Mobasher P, Ekelem C, Mesinkovska NA (April 2018). "A Systematic Review of Topical Finasteride in the Treatment of Androgenetic Alopecia in Men and Women". J Drugs Dermatol. 17 (4): 457–463. PMC 6609098. PMID 29601622.
- Marks DH, Prasad S, De Souza B, Burns LJ, Senna MM (December 2019). "Topical Antiandrogen Therapies for Androgenetic Alopecia and Acne Vulgaris". Am J Clin Dermatol. 21 (2): 245–254. doi:10.1007/s40257-019-00493-z. PMID 31832993.
- F. William Danby (27 January 2015). Acne: Causes and Practical Management. John Wiley & Sons. pp. 147–. ISBN 978-1-118-23277-4.
- Marchetti PM, Barth JH (March 2013). "Clinical biochemistry of dihydrotestosterone". Ann. Clin. Biochem. 50 (Pt 2): 95–107. doi:10.1258/acb.2012.012159. PMID 23431485.
- Hu AC, Chapman LW, Mesinkovska NA (January 2019). "The efficacy and use of finasteride in women: a systematic review". Int. J. Dermatol. 58 (7): 759–776. doi:10.1111/ijd.14370. PMID 30604525.
- Alikhan A, Lynch PJ, Eisen DB (April 2009). "Hidradenitis suppurativa: a comprehensive review". J. Am. Acad. Dermatol. 60 (4): 539–61, quiz 562–3. doi:10.1016/j.jaad.2008.11.911. PMID 19293006.
- Riis PT, Ring HC, Themstrup L, Jemec GB (December 2016). "The Role of Androgens and Estrogens in Hidradenitis Suppurativa - A Systematic Review". Acta Dermatovenerol Croat. 24 (4): 239–249. PMID 28128074.