Predation
Predation is a biological interaction where one organism, the predator, kills and eats another organism, its prey. It is one of a family of common feeding behaviours that includes parasitism and micropredation (which usually do not kill the host) and parasitoidism (which always does, eventually). It is distinct from scavenging on dead prey, though many predators also scavenge; it overlaps with herbivory, as seed predators and destructive frugivores are predators.
_with_its_prey.jpg)
Predators may actively search for or pursue prey or wait for it, often concealed. When prey is detected, the predator assesses whether to attack it. This may involve ambush or pursuit predation, sometimes after stalking the prey. If the attack is successful, the predator kills the prey, removes any inedible parts like the shell or spines, and eats it.
Predators are adapted and often highly specialized for hunting, with acute senses such as vision, hearing, or smell. Many predatory animals, both vertebrate and invertebrate, have sharp claws or jaws to grip, kill, and cut up their prey. Other adaptations include stealth and aggressive mimicry that improve hunting efficiency.
Predation has a powerful selective effect on prey, and the prey develop antipredator adaptations such as warning coloration, alarm calls and other signals, camouflage, mimicry of well-defended species, and defensive spines and chemicals. Sometimes predator and prey find themselves in an evolutionary arms race, a cycle of adaptations and counter-adaptations. Predation has been a major driver of evolution since at least the Cambrian period.
Definition
At the most basic level, predators kill and eat other organisms. However, the concept of predation is broad, defined differently in different contexts, and includes a wide variety of feeding methods; and some relationships that result in the prey's death are not generally called predation. A parasitoid, such as an ichneumon wasp, lays its eggs in or on its host; the eggs hatch into larvae, which eat the host, and it inevitably dies. Zoologists generally call this a form of parasitism, though conventionally parasites are thought not to kill their hosts. A predator can be defined to differ from a parasitoid in that it has many prey, captured over its lifetime, where a parasitoid's larva has just one, or at least has its food supply provisioned for it on just one occasion.[1][2]

There are other difficult and borderline cases. Micropredators are small animals that, like predators, feed entirely on other organisms; they include fleas and mosquitoes that consume blood from living animals, and aphids that consume sap from living plants. However, since they typically do not kill their hosts, they are now often thought of as parasites.[3][4] Animals that graze on phytoplankton or mats of microbes are predators, as they consume and kill their food organisms; but herbivores that browse leaves are not, as their food plants usually survive the assault.[5] When animals eat seeds (seed predation or granivory) or eggs (egg predation), they are consuming entire living organisms, which by definition makes them predators.[6][7][8][6]
Scavengers, organisms that only eat organisms found already dead, are not predators, but many predators such as the jackal and the hyena scavenge when the opportunity arises.[9][10][5] Among invertebrates, social wasps (yellowjackets) are both hunters and scavengers of other insects.[11]
Taxonomic range

While examples of predators among mammals and birds are well known,[12] predators can be found in a broad range of taxa including arthropods. They are common among insects, including mantids, dragonflies, lacewings and scorpionflies. In some species such as the alderfly, only the larvae are predatory (the adults do not eat). Spiders are predatory, as well as other terrestrial invertebrates such as scorpions; centipedes; some mites, snails and slugs; nematodes; and planarian worms.[13] In marine environments, most cnidarians (e.g., jellyfish, hydroids), ctenophora (comb jellies), echinoderms (e.g., sea stars, sea urchins, sand dollars, and sea cucumbers) and flatworms are predatory.[14] Among crustaceans, lobsters, crabs, shrimps and barnacles are predators,[15] and in turn crustaceans are preyed on by nearly all cephalopods (including octopuses, squid and cuttlefish).[16] Arthropods have also been found to be a common predator to a wide range of vertebrates such as amphibians, reptiles, birds, fish, and mammals.[17]
Seed predation is restricted to mammals, birds, and insects and is found in almost all terrestrial ecosystems.[8][6] Egg predation includes both specialist egg predators such as some colubrid snakes and generalists such as foxes and badgers that opportunistically take eggs when they find them.[18][19][20]
Some plants, like the pitcher plant, the Venus fly trap and the sundew, are carnivorous and consume insects.[12] Some carnivorous fungi catch nematodes using either active traps in the form of constricting rings, or passive traps with adhesive structures.[21]
Many species of protozoa (eukaryotes) and bacteria (prokaryotes) prey on other microorganisms; the feeding mode is evidently ancient, and evolved many times in both groups.[22][12][23] Among freshwater and marine zooplankton, whether single-celled or multi-cellular, predatory grazing on phytoplankton and smaller zooplankton is common, and found in many species of nanoflagellates, dinoflagellates, ciliates, rotifers, a diverse range of meroplankton animal larvae, and two groups of crustaceans, namely copepods and cladocerans.[24]
Foraging
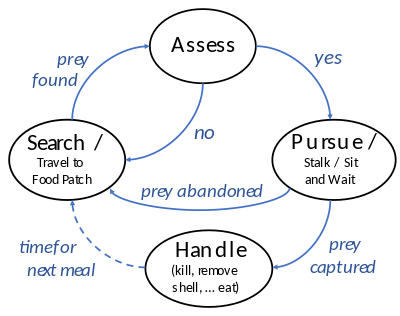
To feed, a predator must search for, pursue and kill its prey. These actions form a foraging cycle.[26][27] The predator must decide where to look for prey based on its geographical distribution; and once it has located prey, it must assess whether to pursue it or to wait for a better choice. If it chooses pursuit, its physical capabilities determine the mode of pursuit (e.g., ambush or chase).[28][29] Having captured the prey, it may also need to expend energy handling it (e.g., killing it, removing any shell or spines, and ingesting it).[25][26]
Search
Predators have a choice of search modes ranging from sit-and-wait to active or widely foraging.[30][25][31][32] The sit-and-wait method is most suitable if the prey are dense and mobile, and the predator has low energy requirements.[30] Wide foraging expends more energy, and is used when prey is sedentary or sparsely distributed.[28][30] There is a continuum of search modes with intervals between periods of movement ranging from seconds to months. Sharks, sunfish, Insectivorous birds and shrews are almost always moving while web-building spiders, aquatic invertebrates, praying mantises and kestrels rarely move. In between, plovers and other shorebirds, freshwater fish including crappies, and the larvae of coccinellid beetles (ladybirds), alternate between actively searching and scanning the environment.[30]

Prey distributions are often clumped, and predators respond by looking for patches where prey is dense and then searching within patches.[25] Where food is found in patches, such as rare shoals of fish in a nearly empty ocean, the search stage requires the predator to travel for a substantial time, and to expend a significant amount of energy, to locate each food patch.[33] For example, the black-browed albatross regularly makes foraging flights to a range of around 700 kilometres (430 miles), up to a maximum foraging range of 3,000 kilometres (1,860 miles) for breeding birds gathering food for their young.[lower-alpha 1][34] With static prey, some predators can learn suitable patch locations and return to them at intervals to feed.[33] The optimal foraging strategy for search has been modelled using the marginal value theorem.[35]
Search patterns often appear random. One such is the Lévy walk, that tends to involve clusters of short steps with occasional long steps. It is a good fit to the behaviour of a wide variety of organisms including bacteria, honeybees, sharks and human hunter-gatherers.[36][37]
Assessment
Having found prey, a predator must decide whether to pursue it or keep searching. The decision depends on the costs and benefits involved. A bird foraging for insects spends a lot of time searching but capturing and eating them is quick and easy, so the efficient strategy for the bird is to eat every palatable insect it finds. By contrast, a predator such as a lion or falcon finds its prey easily but capturing it requires a lot of effort. In that case, the predator is more selective.[28]
One of the factors to consider is size. Prey that is too small may not be worth the trouble for the amount of energy it provides. Too large, and it may be too difficult to capture. For example, a mantid captures prey with its forelegs and they are optimized for grabbing prey of a certain size. Mantids are reluctant to attack prey that is far from that size. There is a positive correlation between the size of a predator and its prey.[28]
A predator may also assess a patch and decide whether to spend time searching for prey in it.[25] This may involve some knowledge of the preferences of the prey; for example, ladybirds can choose a patch of vegetation suitable for their aphid prey.[38]
Capture
To capture prey, predators have a spectrum of pursuit modes that range from overt chase (pursuit predation) to a sudden strike on nearby prey (ambush predation).[25][39][12] Another strategy in between ambush and pursuit is ballistic interception, where a predator observes and predicts a prey's motion and then launches its attack accordingly.[40]
Ambush


Ambush or sit-and-wait predators are carnivorous animals that capture prey by stealth or surprise. In animals, ambush predation is characterized by the predator's scanning the environment from a concealed position until a prey is spotted, and then rapidly executing a fixed surprise attack.[41][40] Vertebrate ambush predators include frogs, fish such as the angel shark, the northern pike and the eastern frogfish.[40][42][43][44] Among the many invertebrate ambush predators are trapdoor spiders on land and mantis shrimps in the sea.[41][45][46] Ambush predators often construct a burrow in which to hide, improving concealment at the cost of reducing their field of vision. Some ambush predators also use lures to attract prey within striking range.[40] The capturing movement has to be rapid to trap the prey, given that the attack is not modifiable once launched.[40]
Ballistic interception
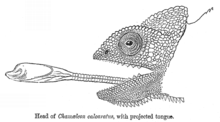
Ballistic interception is the strategy where a predator observes the movement of a prey, predicts its motion, works out an interception path, and then attacks the prey on that path. This differs from ambush predation in that the predator adjusts its attack according to how the prey is moving.[40] Ballistic interception involves a brief period for planning, giving the prey an opportunity to escape. Some frogs wait until snakes have begun their strike before jumping, reducing the time available to the snake to recalibrate its attack, and maximising the angular adjustment that the snake would need to make to intercept the frog in real time.[40] Ballistic predators include insects such as dragonflies, and vertebrates such as archerfish (attacking with a jet of water), chameleons (attacking with their tongues), and some colubrid snakes.[40]
Pursuit


In pursuit predation, predators chase fleeing prey. If the prey flees in a straight line, capture depends only on the predator's being faster than the prey.[40] If the prey manoeuvres by turning as it flees, the predator must react in real time to calculate and follow a new intercept path, such as by parallel navigation, as it closes on the prey.[40] Many pursuit predators use camouflage to approach the prey as close as possible unobserved (stalking) before starting the pursuit.[40] Pursuit predators include terrestrial mammals such as lions, cheetahs, and wolves; marine predators such as dolphins and many predatory fishes, such as tuna;[47][48] predatory birds (raptors) such as falcons; and insects such as dragonflies.[49]
An extreme form of pursuit is endurance or persistence hunting, in which the predator tires out the prey by following it over a long distance, sometimes for hours at a time. The method is used by human hunter-gatherers and in canids such as African wild dogs and domestic hounds. The African wild dog is an extreme persistence predator, tiring out individual prey by following them for many miles at relatively low speed, compared for example to the cheetah's brief high-speed pursuit.[50]
A specialised form of pursuit predation is the lunge feeding of baleen whales. These very large marine predators feed on plankton, especially krill, diving and actively swimming into concentrations of plankton, and then taking a huge gulp of water and filtering it through their feathery baleen plates.[51][52]
Pursuit predators may be social, like the lion and wolf that hunt in groups, or solitary, like the cheetah.[2]
Handling


Once the predator has captured the prey, it has to handle it: very carefully if the prey is dangerous to eat, such as if it possesses sharp or poisonous spines, as in many prey fish. Some catfish such as the Ictaluridae have spines on the back (dorsal) and belly (pectoral) which lock in the erect position; as the catfish thrashes about when captured, these could pierce the predator's mouth, possibly fatally. Some fish-eating birds like the osprey avoid the danger of spines by tearing up their prey before eating it.[53]
Solitary versus social predation
In social predation, a group of predators cooperates to kill prey. This makes it possible to kill creatures larger than those they could overpower singly; for example, hyenas, and wolves collaborate to catch and kill herbivores as large as buffalo, and lions even hunt elephants.[54][55][56] It can also make prey more readily available through strategies like flushing of prey and herding it into a smaller area. For example, when mixed flocks of birds forage, the birds in front flush out insects that are caught by the birds behind. Spinner dolphins form a circle around a school of fish and move inwards, concentrating the fish by a factor of 200.[57] By hunting socially chimpanzees can catch colobus monkeys that would readily escape an individual hunter, while cooperating Harris hawks can trap rabbits.[54][58]
Predators of different species sometimes cooperate to catch prey. In coral reefs, when fish such as the grouper and coral trout spot prey that is inaccessible to them, they signal to giant moray eels, Napoleon wrasses or octopuses. These predators are able to access small crevices and flush out the prey.[59][60] Killer whales have been known to help whalers hunt baleen whales.[61]
Social hunting allows predators to tackle a wider range of prey, but at the risk of competition for the captured food. Solitary predators have more chance of eating what they catch, at the price of increased expenditure of energy to catch it, and increased risk that the prey will escape.[62][63] Ambush predators are often solitary to reduce the risk of becoming prey themselves.[64] Of 245 terrestrial carnivores, 177 are solitary; and 35 of the 37 wild cats are solitary,[65] including the cougar and cheetah.[62][2] However, the solitary cougar does allow other cougars to share in a kill,[66] and the coyote can be either solitary or social.[67] Other solitary predators include the northern pike,[68] wolf spiders and all the thousands of species of solitary wasps among arthropods,[69][70] and many microorganisms and zooplankton.[22][71]
Specialization
Physical adaptations
Under the pressure of natural selection, predators have evolved a variety of physical adaptations for detecting, catching, killing, and digesting prey. These include speed, agility, stealth, sharp senses, claws, teeth, filters, and suitable digestive systems.[72]
For detecting prey, predators have well-developed vision, smell, or hearing.[12] Predators as diverse as owls and jumping spiders have forward-facing eyes, providing accurate binocular vision over a relatively narrow field of view, whereas prey animals often have less acute all-round vision. Animals such as foxes can smell their prey even when it is concealed under 2 feet (60 cm) of snow or earth. Many predators have acute hearing, and some such as echolocating bats hunt exclusively by active or passive use of sound.[73]
Predators including big cats, birds of prey, and ants share powerful jaws, sharp teeth, or claws which they use to seize and kill their prey. Some predators such as snakes and fish-eating birds like herons and cormorants swallow their prey whole; some snakes can unhinge their jaws to allow them to swallow large prey, while fish-eating birds have long spear-like beaks that they use to stab and grip fast-moving and slippery prey.[73] Fish and other predators have developed the ability to crush or open the armoured shells of molluscs.[74]
Many predators are powerfully built and can catch and kill animals larger than themselves; this applies as much to small predators such as ants and shrews as to big and visibly muscular carnivores like the cougar and lion.[73][2][75]
- Skull of brown bear has large pointed canines for killing prey, and self-sharpening carnassial teeth at rear for cutting flesh with a scissor-like action
 Large compound eyes, sensitive antennae, and powerful jaws (mandibles) of jack jumper ant
Large compound eyes, sensitive antennae, and powerful jaws (mandibles) of jack jumper ant Crab spider, an ambush predator with forward-facing eyes, catching another predator, a field digger wasp
Crab spider, an ambush predator with forward-facing eyes, catching another predator, a field digger wasp.jpg) Red-tailed hawk uses sharp hooked claws and beak to kill and tear up its prey
Red-tailed hawk uses sharp hooked claws and beak to kill and tear up its prey- Specialist: a great blue heron with a speared fish
 Indian python unhinges its jaw to swallow large prey like this chital
Indian python unhinges its jaw to swallow large prey like this chital
Diet and behaviour

.jpg)
Predators are often highly specialized in their diet and hunting behaviour; for example, the Eurasian lynx only hunts small ungulates.[76] Others such as leopards are more opportunistic generalists, preying on at least 100 species.[77][78] The specialists may be highly adapted to capturing their preferred prey, whereas generalists may be better able to switch to other prey when a preferred target is scarce. When prey have a clumped (uneven) distribution, the optimal strategy for the predator is predicted to be more specialized as the prey are more conspicuous and can be found more quickly;[79] this appears to be correct for predators of immobile prey, but is doubtful with mobile prey.[80]
In size-selective predation, predators select prey of a certain size.[81] Large prey may prove troublesome for a predator, while small prey might prove hard to find and in any case provide less of a reward. This has led to a correlation between the size of predators and their prey. Size may also act as a refuge for large prey. For example, adult elephants are relatively safe from predation by lions, but juveniles are vulnerable.[82]
Camouflage and mimicry
.jpg)
.jpg)
Members of the cat family such as the snow leopard (treeless highlands), tiger (grassy plains, reed swamps), ocelot (forest), fishing cat (waterside thickets), and lion (open plains) are camouflaged with coloration and disruptive patterns suiting their habitats.[83]
In aggressive mimicry, certain predators, including insects and fishes, make use of coloration and behaviour to attract prey. Female Photuris fireflies, for example, copy the light signals of other species, thereby attracting male fireflies, which they capture and eat.[84] Flower mantises are ambush predators; camouflaged as flowers, such as orchids, they attract prey and seize it when it is close enough.[85] Frogfishes are extremely well camouflaged, and actively lure their prey to approach using an esca, a bait on the end of a rod-like appendage on the head, which they wave gently to mimic a small animal, gulping the prey in an extremely rapid movement when it is within range.[86]
Venom
Many smaller predators such as the box jellyfish use venom to subdue their prey,[87] and venom can also aid in digestion (as is the case for rattlesnakes and some spiders).[88][89] The marbled sea snake that has adapted to egg predation has atrophied venom glands, and the gene for its three finger toxin contains a mutation (the deletion of two nucleotides) that inactives it. These changes are explained by the fact that its prey does not need to be subdued.[90]
Electric fields

Several groups of predatory fish have the ability to detect, track, and sometimes, as in the electric ray, to incapacitate their prey by generating electric fields using electric organs.[91][92][93] The electric organ is derived from modified nerve or muscle tissue.[94]
Physiology
Physiological adaptations to predation include the ability of predatory bacteria to digest the complex peptidoglycan polymer from the cell walls of the bacteria that they prey upon.[23] Carnivorous vertebrates of all five major classes (fishes, amphibians, reptiles, birds, and mammals) have lower relative rates of sugar to amino acid transport than either herbivores or omnivores, presumably because they acquire plenty of amino acids from the animal proteins in their diet.[95]
Antipredator adaptations
To counter predation, prey have a great variety of defences. They can try to avoid detection. They can detect predators and warn others of their presence. If detected, they can try to avoid being the target of an attack, for example, by signalling that a chase would be unprofitable or by forming groups. If they become a target, they can try to fend off the attack with defences such as armour, quills, unpalatability or mobbing; and they can escape an attack in progress by startling the predator, shedding body parts such as tails, or simply fleeing.[96][97][12][98]
Avoiding detection
Prey can avoid detection by predators with morphological traits and coloration that make them hard to detect. They can also adopt behaviour that avoids predators by, for example, avoiding the times and places where predators forage.[99]
Misdirection


Prey animals make use of a variety of mechanisms including camouflage and mimicry to misdirect the visual sensory mechanisms of predators, enabling the prey to remain unrecognized for long enough to give it an opportunity to escape. Camouflage delays recognition through coloration, shape, and pattern.[73][100] Among the many mechanisms of camouflage are countershading[83] and disruptive coloration.[101] The resemblance can be to the biotic or non-living environment, such as a mantis resembling dead leaves, or to other organisms. In mimicry, an organism has a similar appearance to another species, as in the drone fly, which resembles a bee yet has no sting.[102]
Behavioural mechanisms

Animals avoid predators with behavioural mechanisms such as changing their habitats (particularly when raising young), reducing their activity, foraging less and forgoing reproduction when they sense that predators are about.[103]
Eggs and nestlings are particularly vulnerable to predation, so birds take measures to protect their nests.[99] Where birds locate their nests can have a large effect on the frequency of predation. It is lowest for those such as woodpeckers that excavate their own nests and progressively higher for those on the ground, in canopies and in shrubs.[104] To compensate, shrub nesters must have more broods and shorter nesting times. Birds also choose appropriate habitat (e.g., thick foliage or islands) and avoid forest edges and small habitats. Similarly, some mammals raise their young in dens.[103]
By forming groups, prey can often reduce the frequency of encounters with predators because the visibility of a group does not rise in proportion to its size. However, there are exceptions: for example, human fishermen can only detect large shoals of fish with sonar.[105]
Detecting predators
Recognition
Prey species use sight, sound and odor to detect predators, and they can be quite discriminating. For example, Belding's ground squirrel can distinguish several aerial and ground predators from each other and from harmless species. Prey also distinguish between the calls of predators and non-predators. Some species can even distinguish between dangerous and harmless predators of the same species. In the northeastern Pacific Ocean, transient killer whales prey on seals, but the local killer whales only eat fish. Seals rapidly exit the water if they hear calls between transients. Prey are also more vigilant if they smell predators.[106]
The abilities of prey to detect predators do have limits. Belding's ground squirrel cannot distinguish between harriers flying at different heights, although only the low-flying birds are a threat.[106] Wading birds sometimes take flight when there does not appear to be any predator present. Although such false alarms waste energy and lose feeding time, it can be fatal to make the opposite mistake of taking a predator for a harmless animal.[107]
Vigilance
Prey must remain vigilant, scanning their surroundings for predators. This makes it more difficult to feed and sleep. Groups can provide more eyes, making detection of a predator more likely and reducing the level of vigilance needed by individuals.[108] Many species, such as Eurasian jays, give alarm calls warning of the presence of a predator; these give other prey of the same or different species an opportunity to escape, and signal to the predator that it has been detected.[109][110]
Avoiding an attack
Signalling unprofitability
.jpg)
If predator and prey have spotted each other, the prey can signal to the predator to decrease the likelihood of an attack. These honest signals may benefit both the prey and predator, because they save the effort of a fruitless chase.[111] Signals that appear to deter attacks include stotting, for example by Thomson's gazelle;[112][111] push-up displays by lizards;[111] and good singing by skylarks after a pursuit begins.[111] Simply indicating that the predator has been spotted, as a hare does by standing on its hind legs and facing the predator, may sometimes be sufficient.[111]
Many prey animals are aposematically coloured or patterned as a warning to predators that they are distasteful or able to defend themselves.[73][113][114] Such distastefulness or toxicity is brought about by chemical defences, found in a wide range of prey, especially insects, but the skunk is a dramatic mammalian example.[115]
Forming groups
By forming groups, prey can reduce attacks by predators. There are several mechanisms that produce this effect. One is dilution, where, in the simplest scenario, if a given predator attacks a group of prey, the chances of a given individual being the target is reduced in proportion to the size of the group. However, it is difficult to separate this effect from other group-related benefits such as increased vigilance and reduced encounter rate.[116][117] Other advantages include confusing predators such as with motion dazzle, making it more difficult to single out a target.[118][119]
Fending off an attack

Chemical defences include toxins, such as bitter compounds in leaves absorbed by leaf-eating insects, are used to dissuade potential predators.[120] Mechanical defences include sharp spines, hard shells and tough leathery skin or exoskeletons, all making prey harder to kill.[121]
Some species mob predators cooperatively, reducing the likelihood of attack.[122]
Escaping an attack
When a predator is approaching an individual and attack seems imminent, the prey still has several options. One is to flee, whether by running, jumping, climbing, burrowing or swimming.[123] The prey can gain some time by startling the predator. Many butterflies and moths have eyespots, wing markings that resemble eyes.[124] When a predator disturbs the insect, it reveals its hind wings in a deimatic or bluffing display, startling the predator and giving the insect time to escape.[125][126] Some other strategies include playing dead and uttering a distress call.[123]
Coevolution

Predators and prey are natural enemies, and many of their adaptations seem designed to counter each other. For example, bats have sophisticated echolocation systems to detect insects and other prey, and insects have developed a variety of defences including the ability to hear the echolocation calls.[127][128] Many pursuit predators that run on land, such as wolves, have evolved long limbs in response to the increased speed of their prey.[129] Their adaptations have been characterized as an evolutionary arms race, an example of the coevolution of two species.[130] In a gene centered view of evolution, the genes of predator and prey can be thought of as competing for the prey's body.[130] However, the "life-dinner" principle of Dawkins and Krebs predicts that this arms race is asymmetric: if a predator fails to catch its prey, it loses its dinner, while if it succeeds, the prey loses its life.[130]

The metaphor of an arms race implies ever-escalating advances in attack and defence. However, these adaptations come with a cost; for instance, longer legs have an increased risk of breaking,[131] while the specialized tongue of the chameleon, with its ability to act like a projectile, is useless for lapping water, so the chameleon must drink dew off vegetation.[132]
The "life-dinner" principle has been criticized on multiple grounds. The extent of the asymmetry in natural selection depends in part on the heritability of the adaptive traits.[132] Also, if a predator loses enough dinners, it too will lose its life.[131][132] On the other hand, the fitness cost of a given lost dinner is unpredictable, as the predator may quickly find better prey. In addition, most predators are generalists, which reduces the impact of a given prey adaption on a predator. Since specialization is caused by predator-prey coevolution, the rarity of specialists may imply that predator-prey arms races are rare.[132]
It is difficult to determine whether given adaptations are truly the result of coevolution, where a prey adaptation gives rise to a predator adaptation that is countered by further adaptation in the prey. An alternative explanation is escalation, where predators are adapting to competitors, their own predators or dangerous prey.[133] Apparent adaptations to predation may also have arisen for other reasons and then been co-opted for attack or defence. In some of the insects preyed on by bats, hearing evolved before bats appeared and was used to hear signals used for territorial defence and mating.[134] Their hearing evolved in response to bat predation, but the only clear example of reciprocal adaptation in bats is stealth echolocation.[135]
A more symmetric arms race may occur when the prey are dangerous, having spines, quills, toxins or venom that can harm the predator. The predator can respond with avoidance, which in turn drives the evolution of mimicry. Avoidance is not necessarily an evolutionary response as it is generally learned from bad experiences with prey. However, when the prey is capable of killing the predator (as can a coral snake with its venom), there is no opportunity for learning and avoidance must be inherited. Predators can also respond to dangerous prey with counter-adaptations. In western North America, the common garter snake has developed a resistance to the toxin in the skin of the rough-skinned newt.[132]
Role in ecosystems
Trophic level

One way of classifying predators is by trophic level. Carnivores that feed on herbivores are secondary consumers; their predators are tertiary consumers, and so forth.[136] At the top of this food chain are apex predators such as lions.[137] Many predators however eat from multiple levels of the food chain; a carnivore may eat both secondary and tertiary consumers.[138] This means that many predators must contend with intraguild predation, where other predators kill and eat them. For example, coyotes compete with and sometimes kill gray foxes and bobcats.[139]
Biodiversity maintained by apex predation
Predators may increase the biodiversity of communities by preventing a single species from becoming dominant. Such predators are known as keystone species and may have a profound influence on the balance of organisms in a particular ecosystem.[140] Introduction or removal of this predator, or changes in its population density, can have drastic cascading effects on the equilibrium of many other populations in the ecosystem. For example, grazers of a grassland may prevent a single dominant species from taking over.[141]
_horiz.jpg)
The elimination of wolves from Yellowstone National Park had profound impacts on the trophic pyramid. In that area, wolves are both keystone species and apex predators. Without predation, herbivores began to over-graze many woody browse species, affecting the area's plant populations. In addition, wolves often kept animals from grazing near streams, protecting the beavers' food sources. The removal of wolves had a direct effect on the beaver population, as their habitat became territory for grazing. Increased browsing on willows and conifers along Blacktail Creek due to a lack of predation caused channel incision because the reduced beaver population was no longer able to slow the water down and keep the soil in place. The predators were thus demonstrated to be of vital importance in the ecosystem.[142]
Population dynamics
_(20330990750).jpg)
In the absence of predators, the population of a species can grow exponentially until it approaches the carrying capacity of the environment.[143] Predators limit the growth of prey both by consuming them and by changing their behavior.[144] Increases or decreases in the prey population can also lead to increases or decreases in the number of predators, for example, through an increase in the number of young they bear.
Cyclical fluctuations have been seen in populations of predator and prey, often with offsets between the predator and prey cycles. A well-known example is that of the snowshoe hare and lynx. Over a broad span of boreal forests in Alaska and Canada, the hare populations fluctuate in near synchrony with a 10-year period, and the lynx populations fluctuate in response. This was first seen in historical records of animals caught by fur hunters for the Hudson Bay Company over more than a century.[145][146][147][148]
.png)
A simple model of a system with one species each of predator and prey, the Lotka–Volterra equations, predicts population cycles.[149] However, attempts to reproduce the predictions of this model in the laboratory have often failed; for example, when the protozoan Didinium nasutum is added to a culture containing its prey, Paramecium caudatum, the latter is often driven to extinction.[150]
The Lotka-Volterra equations rely on several simplifying assumptions, and they are structurally unstable, meaning that any change in the equations can stabilize or destabilize the dynamics.[151][152] For example, one assumption is that predators have a linear functional response to prey: the rate of kills increases in proportion to the rate of encounters. If this rate is limited by time spent handling each catch, then prey populations can reach densities above which predators cannot control them.[150] Another assumption is that all prey individuals are identical. In reality, predators tend to select young, weak, and ill individuals, leaving prey populations able to regrow.[153]
Many factors can stabilize predator and prey populations.[154] One example is the presence of multiple predators, particularly generalists that are attracted to a given prey species if it is abundant and look elsewhere if it is not.[155] As a result, population cycles tend to be found in northern temperate and subarctic ecosystems because the food webs are simpler.[156] The snowshoe hare-lynx system is subarctic, but even this involves other predators, including coyotes, goshawks and great horned owls, and the cycle is reinforced by variations in the food available to the hares.[157]
A range of mathematical models have been developed by relaxing the assumptions made in the Lotka-Volterra model; these variously allow animals to have geographic distributions, or to migrate; to have differences between individuals, such as sexes and an age structure, so that only some individuals reproduce; to live in a varying environment, such as with changing seasons;[158][159] and analysing the interactions of more than just two species at once. Such models predict widely differing and often chaotic predator-prey population dynamics.[158][160] The presence of refuge areas, where prey are safe from predators, may enable prey to maintain larger populations but may also destabilize the dynamics.[161][162][163][164]
Evolutionary history
Predation dates from before the rise of commonly recognized carnivores by hundreds of millions (perhaps billions) of years. Predation has evolved repeatedly in different groups of organisms.[5][165] The rise of eukaryotic cells at around 2.7 Gya, the rise of multicellular organisms at about 2 Gya, and the rise of mobile predators (around 600 Mya - 2 Gya, probably around 1 Gya) have all been attributed to early predatory behavior, and many very early remains show evidence of boreholes or other markings attributed to small predator species.[5] It likely triggered major evolutionary transitions including the arrival of cells, eukaryotes, sexual reproduction, multicellularity, increased size, mobility (including insect flight[166]) and armoured shells and exoskeletons.[5]
The earliest predators were microbial organisms, which engulfed or grazed on others. Because the fossil record is poor, these first predators could date back anywhere between 1 and over 2.7 Gya (billion years ago).[5] Predation visibly became important shortly before the Cambrian period—around 550 million years ago—as evidenced by the almost simultaneous development of calcification in animals and algae,[167] and predation-avoiding burrowing. However, predators had been grazing on micro-organisms since at least 1,000 million years ago,[5][168][169] with evidence of selective (rather than random) predation from a similar time.[170]
The fossil record demonstrates a long history of interactions between predators and their prey from the Cambrian period onwards, showing for example that some predators drilled through the shells of bivalve and gastropod molluscs, while others ate these organisms by breaking their shells.[171] Among the Cambrian predators were invertebrates like the anomalocaridids with appendages suitable for grabbing prey, large compound eyes and jaws made of a hard material like that in the exoskeleton of an insect.[172] Some of the first fish to have jaws were the armoured and mainly predatory placoderms of the Silurian to Devonian periods, one of which, the 6 m (20 ft) Dunkleosteus, is considered the world's first vertebrate "superpredator", preying upon other predators.[173][174] Insects developed the ability to fly in the Early Carboniferous or Late Devonian, enabling them among other things to escape from predators.[166] Among the largest predators that have ever lived were the theropod dinosaurs such as Tyrannosaurus from the Cretaceous period. They preyed upon herbivorous dinosaurs such as hadrosaurs, ceratopsians and ankylosaurs.[175]
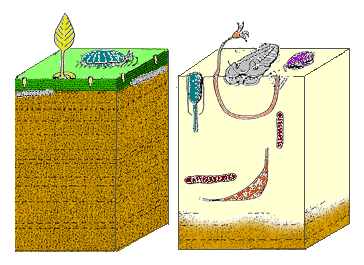 The Cambrian substrate revolution saw life on the sea floor change from minimal burrowing (left) to a diverse burrowing fauna (right), probably to avoid new Cambrian predators.
The Cambrian substrate revolution saw life on the sea floor change from minimal burrowing (left) to a diverse burrowing fauna (right), probably to avoid new Cambrian predators. Mouth of the anomalocaridid Laggania cambria, a Cambrian invertebrate, probably an apex predator
Mouth of the anomalocaridid Laggania cambria, a Cambrian invertebrate, probably an apex predator Dunkleosteus, a Devonian placoderm, perhaps the world's first vertebrate superpredator, reconstruction
Dunkleosteus, a Devonian placoderm, perhaps the world's first vertebrate superpredator, reconstruction Meganeura monyi, a predatory Carboniferous insect related to dragonflies, could fly to escape terrestrial predators. Its large size, with a wingspan of 65 cm (30 in), may reflect the lack of vertebrate aerial predators at that time.
Meganeura monyi, a predatory Carboniferous insect related to dragonflies, could fly to escape terrestrial predators. Its large size, with a wingspan of 65 cm (30 in), may reflect the lack of vertebrate aerial predators at that time. Tyrannosaurus, a large theropod dinosaur of the Cretaceous, reconstruction
Tyrannosaurus, a large theropod dinosaur of the Cretaceous, reconstruction
In human society

Practical uses
Humans, as omnivores, are to some extent predatory,[176] using weapons and tools to fish,[177] hunt and trap animals.[178] They also use other predatory species such as dogs, cormorants,[179] and falcons to catch prey for food or for sport.[180] Two mid-sized predators, dogs and cats, are the animals most often kept as pets in western societies.[181][182] Human hunters, including the San of southern Africa, use persistence hunting, a form of pursuit predation where the pursuer may be slower than prey such as a kudu antelope over short distances, but follows it in the midday heat until it is exhausted, a pursuit that can take up to five hours.[183][184]
In biological pest control, predators (and parasitoids) from a pest's natural range are introduced to control populations, at the risk of causing unforeseen problems. Natural predators, provided they do no harm to non-pest species, are an environmentally friendly and sustainable way of reducing damage to crops and an alternative to the use of chemical agents such as pesticides.[185]
Symbolic uses

In film, the idea of the predator as a dangerous if humanoid enemy is used in the 1987 science fiction horror action film Predator and its three sequels.[186][187] A terrifying predator, a gigantic man-eating great white shark, is central, too, to Steven Spielberg's 1974 thriller Jaws.[188]
Among poetry on the theme of predation, a predator's consciousness might be explored, such as in Ted Hughes's Pike.[189] The phrase "Nature, red in tooth and claw" from Alfred, Lord Tennyson's 1849 poem "In Memoriam A.H.H." has been interpreted as referring to the struggle between predators and prey.[190]
In mythology and folk fable, predators such as the fox and wolf have mixed reputations.[191] The fox was a symbol of fertility in ancient Greece, but a weather demon in northern Europe, and a creature of the devil in early Christianity; the fox is presented as sly, greedy, and cunning in fables from Aesop onwards.[191] The big bad wolf is known to children in tales such as Little Red Riding Hood, but is a demonic figure in the Icelandic Edda sagas, where the wolf Fenrir appears in the apocalyptic ending of the world.[191] In the Middle Ages, belief spread in werewolves, men transformed into wolves.[191] In ancient Rome, and in ancient Egypt, the wolf was worshipped, the she-wolf appearing in the founding myth of Rome, suckling Romulus and Remus.[191] More recently, in Rudyard Kipling's 1894 The Jungle Book, Mowgli is raised by the wolf pack.[191] Attitudes to large predators in North America, such as wolf, grizzly bear and cougar, have shifted from hostility or ambivalence, accompanied by active persecution, towards positive and protective in the second half of the 20th century.[192]
Notes
- A range of 3000 kilometres means a flight distance of at least 6000 kilometres out and back.
References
- Gurr, Geoff M.; Wratten, Stephen D.; Snyder, William E. (2012). Biodiversity and Insect Pests: Key Issues for Sustainable Management. John Wiley & Sons. p. 105. ISBN 978-1-118-23185-2.
- Lafferty, K. D.; Kuris, A. M. (2002). "Trophic strategies, animal diversity and body size". Trends Ecol. Evol. 17 (11): 507–513. doi:10.1016/s0169-5347(02)02615-0.
- Poulin, Robert; Randhawa, Haseeb S. (February 2015). "Evolution of parasitism along convergent lines: from ecology to genomics". Parasitology. 142 (Suppl 1): S6–S15. doi:10.1017/S0031182013001674. PMC 4413784. PMID 24229807.
- Poulin, Robert (2011). Rollinson, D.; Hay, S. I. (eds.). The Many Roads to Parasitism: A Tale of Convergence. Advances in Parasitology. 74. Academic Press. pp. 27–28. doi:10.1016/B978-0-12-385897-9.00001-X. ISBN 978-0-12-385897-9. PMID 21295676.
- Bengtson, S. (2002). "Origins and early evolution of predation". In Kowalewski, M.; Kelley, P. H. (eds.). The fossil record of predation. The Paleontological Society Papers 8 (PDF). The Paleontological Society. pp. 289–317.
- Janzen, D. H. (1971). "Seed Predation by Animals". Annual Review of Ecology and Systematics. 2: 465–492. doi:10.1146/annurev.es.02.110171.002341.
- Nilsson, Sven G.; Björkman, Christer; Forslund, Pär; Höglund, Jacob (1985). "Egg predation in forest bird communities on islands and mainland". Oecologia. 66 (4): 511–515. Bibcode:1985Oecol..66..511N. doi:10.1007/BF00379342. PMID 28310791.
- Hulme, P. E.; Benkman, C. W. (2002). C. M. Herrera and O. Pellmyr (ed.). Granivory. Plant animal Interactions: An Evolutionary Approach. Blackwell. pp. 132–154. ISBN 978-0-632-05267-7.
- Kane, Adam; Healy, Kevin; Guillerme, Thomas; Ruxton, Graeme D.; Jackson, Andrew L. (2017). "A recipe for scavenging in vertebrates – the natural history of a behaviour". Ecography. 40 (2): 324–334. doi:10.1111/ecog.02817. hdl:10468/3213.
- Kruuk, Hans (1972). The Spotted Hyena: A Study of Predation and Social Behaviour. University of California Press. pp. 107–108. ISBN 978-0226455082.
- Schmidt, Justin O. (2009). Wasps. Encyclopedia of Insects (Second ed.). pp. 1049–1052. doi:10.1016/B978-0-12-374144-8.00275-7. ISBN 9780123741448.
- Stevens, Alison N. P. (2010). "Predation, Herbivory, and Parasitism". Nature Education Knowledge. 3 (10): 36.
- "Predators, parasites and parasitoids". Australian Museum. Retrieved 19 September 2018.
- Watanabe, James M. (2007). "Invertebrates, overview". In Denny, Mark W.; Gaines, Steven Dean (eds.). Encyclopedia of tidepools and rocky shores. University of California Press. ISBN 9780520251182.
- Phelan, Jay (2009). What Is life? : a guide to biology (Student ed.). W.H. Freeman & Co. p. 432. ISBN 9781429223188.
- Villanueva, Roger; Perricone, Valentina; Fiorito, Graziano (17 August 2017). "Cephalopods as Predators: A Short Journey among Behavioral Flexibilities, Adaptions, and Feeding Habits". Frontiers in Physiology. 8: 598. doi:10.3389/fphys.2017.00598. PMC 5563153. PMID 28861006.
- Valdez, Jose W. (27 July 2020). Lyons, Kathleen (ed.). "Arthropods as vertebrate predators: A review of global patterns". Global Ecology and Biogeography: geb.13157. doi:10.1111/geb.13157. ISSN 1466-822X.
- Hanssen, Sveinn Are; Erikstad, Kjell Einar (2012). "The long-term consequences of egg predation". Behavioral Ecology. 24 (2): 564–569. doi:10.1093/beheco/ars198.
- Pike, David A.; Clark, Rulon W.; Manica, Andrea; Tseng, Hui-Yun; Hsu, Jung-Ya; Huang, Wen-San (26 February 2016). "Surf and turf: predation by egg-eating snakes has led to the evolution of parental care in a terrestrial lizard". Scientific Reports. 6 (1): 22207. doi:10.1038/srep22207. PMC 4768160. PMID 26915464.
- Ainsworth, Gill; Calladine, John; Martay, Blaise; Park, Kirsty; Redpath, Steve; Wernham, Chris; Wilson, Mark; Young, Juliette (2017). Understanding Predation: A review bringing together natural science and local knowledge of recent wild bird population changes and their drivers in Scotland. Scotland's Moorland Forum. pp. 233–234. doi:10.13140/RG.2.1.1014.6960.
- Pramer, D. (1964). "Nematode-trapping fungi". Science. 144 (3617): 382–388. Bibcode:1964Sci...144..382P. doi:10.1126/science.144.3617.382. JSTOR 1713426. PMID 14169325.
- Velicer, Gregory J.; Mendes-Soares, Helena (2007). "Bacterial predators" (PDF). Cell. 19 (2): R55–R56. doi:10.1016/j.cub.2008.10.043. PMID 19174136.
- Jurkevitch, Edouard; Davidov, Yaacov (2006). "Phylogenetic Diversity and Evolution of Predatory Prokaryotes". Predatory Prokaryotes. Springer. pp. 11–56. doi:10.1007/7171_052. ISBN 978-3-540-38577-6.
- Hansen, Per Juel; Bjørnsen, Peter Koefoed; Hansen, Benni Winding (1997). "Zooplankton grazing and growth: Scaling within the 2-2,-μm body size range". Limnology and Oceanography. 42 (4): 687–704. doi:10.4319/lo.1997.42.4.0687. summarizes findings from many authors.
- Kramer, Donald L. (2001). "Foraging behavior" (PDF). In Fox, C. W.; Roff, D. A.; Fairbairn, D. J. (eds.). Evolutionary Ecology: Concepts and Case Studies. Oxford University Press. pp. 232–238. ISBN 9780198030133.
- Griffiths, David (November 1980). "Foraging costs and relative prey size". The American Naturalist. 116 (5): 743–752. doi:10.1086/283666. JSTOR 2460632.
- Wetzel, Robert G.; Likens, Gene E. (2000). "Predator-Prey Interactions". Limnological Analyses. Springer. pp. 257–262. doi:10.1007/978-1-4757-3250-4_17. ISBN 978-1-4419-3186-3.
- Pianka, Eric R. (2011). Evolutionary ecology (7th (eBook) ed.). Eric R. Pianka. pp. 78–83.
- MacArthur, Robert H. (1984). "The economics of consumer choice". Geographical ecology : patterns in the distribution of species. Princeton University Press. pp. 59–76. ISBN 9780691023823.
- Bell 2012, pp. 4–5
- Eastman, Lucas B.; Thiel, Martin (2015). Thiel, Martin; Watling, Les (eds.). Lifestyles and feeding biology. Oxford University Press. pp. 535–556. ISBN 9780199797066.
- Perry, Gad (January 1999). "The Evolution of Search Modes: Ecological versus Phylogenetic Perspectives". The American Naturalist. 153 (1): 98–109. doi:10.1086/303145. PMID 29578765.
- Bell 2012, pp. 69–188
- Gremillet, D.; Wilson, R. P.; Wanless, S.; Chater, T. (2000). "Black-browed albatrosses, international fisheries and the Patagonian Shelf". Marine Ecology Progress Series. 195: 69–280. doi:10.3354/meps195269.
- Charnov, Eric L. (1976). "Optimal foraging, the marginal value theorem" (PDF). Theoretical Population Biology. 9 (2): 129–136. doi:10.1016/0040-5809(76)90040-x. PMID 1273796.
- Reynolds, Andy (September 2015). "Liberating Lévy walk research from the shackles of optimal foraging". Physics of Life Reviews. 14: 59–83. doi:10.1016/j.plrev.2015.03.002. PMID 25835600.
- Buchanan, Mark (5 June 2008). "Ecological modelling: The mathematical mirror to animal nature". Nature. 453 (7196): 714–716. doi:10.1038/453714a. PMID 18528368.
- Williams, Amanda C.; Flaxman, Samuel M. (2012). "Can predators assess the quality of their prey's resource?". Animal Behaviour. 83 (4): 883–890. doi:10.1016/j.anbehav.2012.01.008.
- Scharf, Inon; Nulman, Einat; Ovadia, Ofer; Bouskila, Amos (September 2006). "Efficiency evaluation of two competing foraging modes under different conditions". The American Naturalist. 168 (3): 350–357. doi:10.1086/506921. PMID 16947110.
- Moore, Talia Y.; Biewener, Andrew A. (2015). "Outrun or Outmaneuver: Predator–Prey Interactions as a Model System for Integrating Biomechanical Studies in a Broader Ecological and Evolutionary Context" (PDF). Integrative and Comparative Biology. 55 (6): 1188–97. doi:10.1093/icb/icv074. PMID 26117833.
- deVries, M. S.; Murphy, E. A. K.; Patek S. N. (2012). "Strike mechanics of an ambush predator: the spearing mantis shrimp". Journal of Experimental Biology. 215 (Pt 24): 4374–4384. doi:10.1242/jeb.075317. PMID 23175528.
- "Cougar". Hinterland Who's Who. Canadian Wildlife Service and Canadian Wildlife Federation. Archived from the original on 18 May 2007. Retrieved 22 May 2007.
- "Pikes (Esocidae)" (PDF). Indiana Division of Fish and Wildlife. Retrieved 3 September 2018.
- Bray, Dianne. "Eastern Frogfish, Batrachomoeus dubius". Fishes of Australia. Archived from the original on 14 September 2014. Retrieved 14 September 2014.
- "Trapdoor spiders". BBC. Retrieved 12 December 2014.
- "Trapdoor spider". Arizona-Sonora Desert Museum. 2014. Retrieved 12 December 2014.
- Gazda, S. K.; Connor, R. C.; Edgar, R. K.; Cox, F. (2005). "A division of labour with role specialization in group-hunting bottlenose dolphins (Tursiops truncatus) off Cedar Key, Florida". Proceedings of the Royal Society. 272 (1559): 135–140. doi:10.1098/rspb.2004.2937. PMC 1634948. PMID 15695203.
- Tyus, Harold M. (2011). Ecology and Conservation of Fishes. CRC Press. p. 233. ISBN 978-1-4398-9759-1.
- Combes, S. A.; Salcedo, M. K.; Pandit, M. M.; Iwasaki, J. M. (2013). "Capture Success and Efficiency of Dragonflies Pursuing Different Types of Prey". Integrative and Comparative Biology. 53 (5): 787–798. doi:10.1093/icb/ict072. PMID 23784698.
- Hubel, Tatjana Y.; Myatt, Julia P.; Jordan, Neil R.; Dewhirst, Oliver P.; McNutt, J. Weldon; Wilson, Alan M. (29 March 2016). "Energy cost and return for hunting in African wild dogs and cheetahs". Nature Communications. 7: 11034. doi:10.1038/ncomms11034. PMC 4820543. PMID 27023457.
Cursorial hunting strategies range from one extreme of transient acceleration, power and speed to the other extreme of persistence and endurance with prey being fatigued to facilitate capture. Cheetahs use high acceleration, speed and manoeuvrability to capture prey in a relatively short chase4. Dogs and humans are considered to rely on endurance rather than outright speed and manoeuvrability for success when hunting cursorially.
- Goldbogen, J. A.; Calambokidis, J.; Shadwick, R. E.; Oleson, E. M.; McDonald, M. A.; Hildebrand, J. A. (2006). "Kinematics of foraging dives and lunge-feeding in fin whales" (PDF). Journal of Experimental Biology. 209 (7): 1231–1244. doi:10.1242/jeb.02135. PMID 16547295.
- Sanders, Jon G.; Beichman, Annabel C.; Roman, Joe; Scott, Jarrod J.; Emerson, David; McCarthy, James J.; Girguis, Peter R. (2015). "Baleen whales host a unique gut microbiome with similarities to both carnivores and herbivores". Nature Communications. 6: 8285. Bibcode:2015NatCo...6.8285S. doi:10.1038/ncomms9285. PMC 4595633. PMID 26393325.
- Forbes, L. Scott (1989). "Prey Defences and Predator Handling Behaviour: The Dangerous Prey Hypothesis". Oikos. 55 (2): 155–158. doi:10.2307/3565418. JSTOR 3565418.
- Lang, Stephen D. J.; Farine, Damien R. (2017). "A multidimensional framework for studying social predation strategies". Nature Ecology & Evolution. 1 (9): 1230–1239. doi:10.1038/s41559-017-0245-0. PMID 29046557.
- MacNulty, Daniel R.; Tallian, Aimee; Stahler, Daniel R.; Smith, Douglas W. (12 November 2014). Sueur, Cédric (ed.). "Influence of Group Size on the Success of Wolves Hunting Bison". PLOS ONE. 9 (11): e112884. Bibcode:2014PLoSO...9k2884M. doi:10.1371/journal.pone.0112884. PMC 4229308. PMID 25389760.
- Power, R. J.; Compion, R. X. Shem (2009). "Lion predation on elephants in the Savuti, Chobe National Park, Botswana". African Zoology. 44 (1): 36–44. doi:10.3377/004.044.0104.
- Beauchamp 2012, pp. 7–12
- Dawson, James W. (1988). "The cooperative breeding system of the Harris' Hawk in Arizona". The University of Arizona. Retrieved 17 November 2017. Cite journal requires
|journal=(help) - Vail, Alexander L.; Manica, Andrea; Bshary, Redouan (23 April 2013). "Referential gestures in fish collaborative hunting". Nature Communications. 4 (1): 1765. Bibcode:2013NatCo...4.1765V. doi:10.1038/ncomms2781. PMID 23612306.
- Yong, Ed (24 April 2013). "Groupers Use Gestures to Recruit Morays For Hunting Team-Ups". National Geographic. Retrieved 17 September 2018.
- Toft, Klaus (Producer) (2007). Killers in Eden (DVD documentary). Australian Broadcasting Corporation. Archived from the original on 12 August 2009. ISBN R-105732-9.
- Bryce, Caleb M.; Wilmers, Christopher C.; Williams, Terrie M. (2017). "Energetics and evasion dynamics of large predators and prey: pumas vs. hounds". PeerJ. 5: e3701. doi:10.7717/peerj.3701. PMC 5563439. PMID 28828280.
- Majer, Marija; Holm, Christina; Lubin, Yael; Bilde, Trine (2018). "Cooperative foraging expands dietary niche but does not offset intra-group competition for resources in social spiders". Scientific Reports. 8 (1): 11828. doi:10.1038/s41598-018-30199-x. PMC 6081395. PMID 30087391.
- "Ambush Predators". Sibley Nature Center. Retrieved 17 September 2018.
- Elbroch, L. Mark; Quigley, Howard (10 July 2016). "Social interactions in a solitary carnivore". Current Zoology. 63 (4): 357–362. doi:10.1093/cz/zow080. PMC 5804185. PMID 29491995.
- Quenqua, Douglas (11 October 2017). "Solitary Pumas Turn Out to Be Mountain Lions Who Lunch". The New York Times. Retrieved 17 September 2018.
- Flores, Dan (2016). Coyote America : a natural and supernatural history. Basic Books. ISBN 978-0465052998.
- Stow, Adam; Nyqvist, Marina J.; Gozlan, Rodolphe E.; Cucherousset, Julien; Britton, J. Robert (2012). "Behavioural Syndrome in a Solitary Predator Is Independent of Body Size and Growth Rate". PLOS ONE. 7 (2): e31619. doi:10.1371/journal.pone.0031619. PMC 3282768. PMID 22363687.
- "How do Spiders Hunt?". American Museum of Natural History. 25 August 2014. Retrieved 5 September 2018.
- Weseloh, Ronald M.; Hare, J. Daniel (2009). "Predation/Predatory Insects". Encyclopedia of Insects (Second ed.). pp. 837–839. doi:10.1016/B978-0-12-374144-8.00219-8. ISBN 9780123741448.
- "Zooplankton". MarineBio Conservation Society. Retrieved 5 September 2018.
- Bar-Yam. "Predator-Prey Relationships". New England Complex Systems Institute. Retrieved 7 September 2018.
- "Predator & Prey: Adaptations" (PDF). Royal Saskatchewan Museum. 2012. Retrieved 19 April 2018.
- Vermeij, Geerat J. (1993). Evolution and Escalation: An Ecological History of Life. Princeton University Press. pp. 11 and passim. ISBN 978-0-691-00080-0.
- Getz, W. M. (2011). "Biomass transformation webs provide a unified approach to consumer-resource modelling". Ecology Letters. 14 (2): 113–24. doi:10.1111/j.1461-0248.2010.01566.x. PMC 3032891. PMID 21199247.
- Sidorovich, Vadim (2011). Analysis of vertebrate predator-prey community: Studies within the European Forest zone in terrains with transitional mixed forest in Belarus. Tesey. p. 426. ISBN 978-985-463-456-2.
- Angelici, Francesco M. (2015). Problematic Wildlife: A Cross-Disciplinary Approach. Springer. p. 160. ISBN 978-3-319-22246-2.
- Hayward, M. W.; Henschel, P.; O'Brien, J.; Hofmeyr, M.; Balme, G.; Kerley, G.I.H. (2006). "Prey preferences of the leopard (Panthera pardus)" (PDF). Journal of Zoology. 270 (2): 298–313. doi:10.1111/j.1469-7998.2006.00139.x.
- Pulliam, H. Ronald (1974). "On the Theory of Optimal Diets". The American Naturalist. 108 (959): 59–74. doi:10.1086/282885.
- Sih, Andrew; Christensen, Bent (2001). "Optimal diet theory: when does it work, and when and why does it fail?". Animal Behaviour. 61 (2): 379–390. doi:10.1006/anbe.2000.1592.
- Sprules, W. Gary (1972). "Effects of Size-Selective Predation and Food Competition on High Altitude Zooplankton Communities". Ecology. 53 (3): 375–386. doi:10.2307/1934223. JSTOR 1934223.
- Owen-Smith, Norman; Mills, M. G. L. (2008). "Predator-prey size relationships in an African large-mammal food web" (PDF). Journal of Animal Ecology. 77 (1): 173–183. doi:10.1111/j.1365-2656.2007.01314.x. hdl:2263/9023. PMID 18177336.
- Cott 1940, pp. 12–13
- Lloyd J. E. (1965). "Aggressive Mimicry in Photuris: Firefly Femmes Fatales". Science. 149 (3684): 653–654. Bibcode:1965Sci...149..653L. doi:10.1126/science.149.3684.653. PMID 17747574.
- Forbes, Peter (2009). Dazzled and Deceived: Mimicry and Camouflage. Yale University Press. p. 134. ISBN 978-0-300-17896-8.
- Bester, Cathleen (5 May 2017). "Antennarius striatus". Florida Museum. University of Florida. Retrieved 31 January 2018.
- Ruppert, Edward E.; Fox, Richard, S.; Barnes, Robert D. (2004). Invertebrate Zoology, 7th edition. Cengage Learning. pp. 153–154. ISBN 978-81-315-0104-7.
- Cetaruk, Edward W. (2005). "Rattlesnakes and Other Crotalids". In Brent, Jeffrey (ed.). Critical care toxicology: diagnosis and management of the critically poisoned patient. Elsevier Health Sciences. p. 1075. ISBN 978-0-8151-4387-1.
- Barceloux, Donald G. (2008). Medical Toxicology of Natural Substances: Foods, Fungi, Medicinal Herbs, Plants, and Venomous Animals. Wiley. p. 1028. ISBN 978-0-470-33557-4.
- Li, Min; Fry, B.G.; Kini, R. Manjunatha (2005). "Eggs-Only Diet: Its Implications for the Toxin Profile Changes and Ecology of the Marbled Sea Snake (Aipysurus eydouxii)". Journal of Molecular Evolution. 60 (1): 81–89. Bibcode:2005JMolE..60...81L. doi:10.1007/s00239-004-0138-0. PMID 15696370.
- Castello, M. E., A. Rodriguez-Cattaneo, P. A. Aguilera, L. Iribarne, A. C. Pereira, and A. A. Caputi (2009). "Waveform generation in the weakly electric fish Gymnotus coropinae (Hoedeman): the electric organ and the electric organ discharge". Journal of Experimental Biology. 212 (9): 1351–1364. doi:10.1242/jeb.022566. PMID 19376956.CS1 maint: multiple names: authors list (link)
- Feulner, P. G., M. Plath, J. Engelmann, F. Kirschbaum, R. Tiedemann (2009). "Electrifying love: electric fish use species-specific discharge for mate recognition". Biology Letters. 5 (2): 225–228. doi:10.1098/rsbl.2008.0566. PMC 2665802. PMID 19033131.CS1 maint: multiple names: authors list (link)
- Catania, Kenneth C. (2015). "Electric eels use high-voltage to track fast-moving prey". Nature Communications. 6 (1): 8638. doi:10.1038/ncomms9638. ISSN 2041-1723. PMC 4667699. PMID 26485580.
- Kramer, Bernd (1996). "Electroreception and communication in fishes" (PDF). Progress in Zoology. 42.
- Karasov, William H.; Diamond, Jared M. (1988). "Interplay between Physiology and Ecology in Digestion". BioScience. 38 (9): 602–611. doi:10.2307/1310825. JSTOR 1310825.
- Caro 2005, pp. v–xi, 4–5
- Ruxton, Sherratt & Speed 2004, pp. vii–xii
- Edmunds, M. (1974). Defence in Animals. Longman. ISBN 978-0582441323.
- Caro 2005, pp. 67–114
- Merilaita, Sami; Scott-Samuel, Nicholas E.; Cuthill, Innes C. (22 May 2017). "How camouflage works". Philosophical Transactions of the Royal Society B: Biological Sciences (Submitted manuscript). 372 (1724): 20160341. doi:10.1098/rstb.2016.0341. PMC 5444062. PMID 28533458.
- Cott 1940, pp. 35–46
- Cott 1940, pp. 396–416
- Caro 2005, pp. 112–113
- Caro 2005, pp. 68–69
- Beauchamp 2012, pp. 78–80
- Caro 2005, pp. 13–15
- Ruxton, Sherratt & Speed 2004, p. 196
- Caro 2005, p. 149
- Bergstrom, C. T.; Lachmann, M. (2001). "Alarm calls as costly signals of antipredator vigilance: the watchful babbler game". Animal Behaviour. 61 (3): 535–543. CiteSeerX 10.1.1.28.773. doi:10.1006/anbe.2000.1636.
- Getty, T. (2002). "The discriminating babbler meets the optimal diet hawk". Anim. Behav. 63 (2): 397–402. doi:10.1006/anbe.2001.1890.
- Ruxton, Sherratt & Speed 2004, pp. 70–81
- Caro 2005, pp. 663–684
- Cott 1940, pp. 241–307
- Bowers, M. D.; Brown, Irene L.; Wheye, Darryl (1985). "Bird Predation as a Selective Agent in a Butterfly Population". Evolution. 39 (1): 93–103. doi:10.1111/j.1558-5646.1985.tb04082.x. PMID 28563638.
- Berenbaum, M. R. (3 January 1995). "The chemistry of defense: theory and practice". Proceedings of the National Academy of Sciences of the United States of America. 92 (1): 2–8. Bibcode:1995PNAS...92....2B. doi:10.1073/pnas.92.1.2. PMC 42807. PMID 7816816.
- Beauchamp 2012, pp. 83–88
- Krause, Jens; Ruxton, Graeme D. (10 October 2002). Living in groups. Oxford University Press. pp. 13–15. ISBN 9780198508182.
- Caro 2005, pp. 275–278
- How, Martin J.; Zanker, Johannes M. (2014). "Motion camouflage induced by zebra stripes" (PDF). Zoology. 117 (3): 163–170. doi:10.1016/j.zool.2013.10.004. PMID 24368147.
- Brodie, Edmund D. (3 November 2009). "Toxins and venoms" (PDF). Current Biology. 19 (20): R931–R935. doi:10.1016/j.cub.2009.08.011. PMID 19889364.
- Ruxton, Sherratt & Speed 2004, pp. 54–55
- Dominey, Wallace J. (1983). "Mobbing in Colonially Nesting Fishes, Especially the Bluegill, Lepomis macrochirus". Copeia. 1983 (4): 1086–1088. doi:10.2307/1445113. JSTOR 1445113.
- Caro 2005, p. 413–414
- Cott 1940, pp. 368–389
- Merilaita, Sami; Vallin, Adrian; Kodandaramaiah, Ullasa; Dimitrova, Marina; Ruuskanen, Suvi; Laaksonen, Toni (26 July 2011). "Number of eyespots and their intimidating effect on naïve predators in the peacock butterfly". Behavioral Ecology. 22 (6): 1326–1331. doi:10.1093/beheco/arr135. Retrieved 27 November 2011.
- Edmunds, Malcolm (2012). "Deimatic Behavior". Springer. Retrieved 31 December 2012.
- Jacobs & Bastian 2017, p. 4
- Barbosa, Pedro; Castellanos, Ignacio (2005). Ecology of predator-prey interactions. Oxford University Press. p. 78. ISBN 9780199874545.
- Janis, C. M.; Wilhelm, P. B. (1993). "Were there mammalian pursuit predators in the Tertiary? Dances with wolf avatars". Journal of Mammalian Evolution. 1 (2): 103–125. doi:10.1007/bf01041590.
- Dawkins, Richard; Krebs, J. R. (1979). "Arms races between and within species". Proceedings of the Royal Society B: Biological Sciences. 205 (1161): 489–511. Bibcode:1979RSPSB.205..489D. doi:10.1098/rspb.1979.0081. PMID 42057.
- Abrams, Peter A. (November 1986). "Adaptive responses of predators to prey and prey to predators: The failure of the arms-race analogy". Evolution. 40 (6): 1229–1247. doi:10.1111/j.1558-5646.1986.tb05747.x. PMID 28563514.
- Brodie, Edmund D.; Brodie, Edmund D. (July 1999). "Predator-Prey Arms Races". BioScience. 49 (7): 557–568. doi:10.2307/1313476. JSTOR 1313476.
- Vermeij, G J (November 1994). "The Evolutionary Interaction Among Species: Selection, Escalation, and Coevolution". Annual Review of Ecology and Systematics. 25 (1): 219–236. doi:10.1146/annurev.es.25.110194.001251.
- Jacobs & Bastian 2017, p. 8
- Jacobs & Bastian 2017, p. 107
- Lindeman, Raymond L. (1942). "The Trophic-Dynamic Aspect of Ecology". Ecology. 23 (4): 399–417. doi:10.2307/1930126. JSTOR 1930126.
- Ordiz, Andrés; Bischof, Richard; Swenson, Jon E. (2013). "Saving large carnivores, but losing the apex predator?". Biological Conservation. 168: 128–133. doi:10.1016/j.biocon.2013.09.024.
- Pimm, S. L.; Lawton, J. H. (1978). "On feeding on more than one trophic level". Nature. 275 (5680): 542–544. Bibcode:1978Natur.275..542P. doi:10.1038/275542a0.
- Fedriani, J. M.; Fuller, T. K.; Sauvajot, R. M.; York, E. C. (2000). "Competition and intraguild predation among three sympatric carnivores". Oecologia. 125 (2): 258–270. Bibcode:2000Oecol.125..258F. doi:10.1007/s004420000448. hdl:10261/54628. PMID 24595837.
- Bond, W. J. (2012). "11. Keystone species". In Schulze, Ernst-Detlef; Mooney, Harold A. (eds.). Biodiversity and Ecosystem Function. Springer. p. 237. ISBN 978-3642580017.
- Botkin, D.; Keller, E. (2003). Environmental Science: Earth as a living planet. John Wiley & Sons. p. 2. ISBN 978-0-471-38914-9.
- Ripple, William J.; Beschta, Robert L. (2004). "Wolves and the Ecology of Fear: Can Predation Risk Structure Ecosystems?". BioScience. 54 (8): 755. doi:10.1641/0006-3568(2004)054[0755:WATEOF]2.0.CO;2.
- Neal, Dick (2004). Introduction to population biology. Cambridge University Press. pp. 68–69. ISBN 9780521532235.
- Nelson, Erik H.; Matthews, Christopher E.; Rosenheim, Jay A. (July 2004). "Predators Reduce Prey Population Growth by Inducing Changes in Prey Behavior". Ecology. 85 (7): 1853–1858. doi:10.1890/03-3109. JSTOR 3450359.
- Krebs, Charles J.; Boonstra, Rudy; Boutin, Stan; Sinclair, A.R.E. (2001). "What Drives the 10-year Cycle of Snowshoe Hares?". BioScience. 51 (1): 25. doi:10.1641/0006-3568(2001)051[0025:WDTYCO]2.0.CO;2.
- Peckarsky, Barbara L.; Abrams, Peter A.; Bolnick, Daniel I.; Dill, Lawrence M.; Grabowski, Jonathan H.; Luttbeg, Barney; Orrock, John L.; Peacor, Scott D.; Preisser, Evan L.; Schmitz, Oswald J.; Trussell, Geoffrey C. (September 2008). "Revisiting the classics: considering nonconsumptive effects in textbook examples of predator–prey interactions". Ecology. 89 (9): 2416–2425. doi:10.1890/07-1131.1. PMID 18831163.
- Krebs, Charley; Myers, Judy (12 July 2014). "The Snowshoe Hare 10-year Cycle – A Cautionary Tale". Ecological rants. University of British Columbia. Retrieved 2 October 2018.
- "Predators and their prey". BBC Bitesize. BBC. Retrieved 7 October 2015.
- Goel, Narendra S.; Maitra, S. C.; Montroll, E. W. (1971). On the Volterra and Other Non-Linear Models of Interacting Populations. Academic Press. ISBN 978-0122874505.
- Levin, Simon A.; Carpenter, Stephen R.; Godfray, H. Charles J.; Kinzig, Ann P.; Loreau, Michel; Losos, Jonathan B.; Walker, Brian; Wilcove, David S. (2009). The Princeton guide to ecology. Princeton University Press. pp. 204–209. ISBN 9781400833023.
- Murdoch, William W.; Briggs, Cheryl J.; Nisbet, Roger M. (2013). Consumer-resource dynamics. Princeton University Press. p. 39. ISBN 9781400847259.
- Nowak, Martin; May, Robert M. (2000). Virus Dynamics : Mathematical Principles of Immunology and Virology. Oxford University Press. p. 8. ISBN 9780191588518.
- Genovart, M.; Negre, N.; Tavecchia, G.; Bistuer, A.; Parpal, L.; Oro, D. (2010). "The young, the weak and the sick: evidence of natural selection by predation". PLOS One. 5 (3): e9774. Bibcode:2010PLoSO...5.9774G. doi:10.1371/journal.pone.0009774. PMC 2841644. PMID 20333305.
- Rockwood 2009, p. 281
- Rockwood 2009, p. 246
- Rockwood 2009, pp. 271–272
- Rockwood 2009, p. 272–273
- Cushing, J. M. (2005). "Book Reviews | Mathematics in population biology, by Horst R. Thiene" (PDF). Bulletin of the American Mathematical Society. 42 (4): 501–505. doi:10.1090/S0273-0979-05-01055-4.
- Thieme, Horst R. (2003). Mathematics in Population Biology. Princeton University Press. ISBN 978-0-691-09291-1.
- Kozlov, Vladimir; Vakulenko, Sergey (3 July 2013). "On chaos in Lotka–Volterra systems: an analytical approach". Nonlinearity. 26 (8): 2299–2314. doi:10.1088/0951-7715/26/8/2299.
- Sih, Andrew (1987). "Prey refuges and predator-prey stability". Theoretical Population Biology. 31: 1–12. doi:10.1016/0040-5809(87)90019-0.
- McNair, James N (1986). "The effects of refuges on predator-prey interactions: A reconsideration". Theoretical Population Biology. 29 (1): 38–63. doi:10.1016/0040-5809(86)90004-3. PMID 3961711.
- Berryman, Alan A.; Hawkins, Bradford A.; Hawkins, Bradford A. (2006). "The refuge as an integrating concept in ecology and evolution". Oikos. 115 (1): 192–196. doi:10.1111/j.0030-1299.2006.15188.x.
- Cressman, Ross; Garay, József (2009). "A predator–prey refuge system: Evolutionary stability in ecological systems". Theoretical Population Biology. 76 (4): 248–57. doi:10.1016/j.tpb.2009.08.005. PMID 19751753.
- Abrams, P. A. (2000). "The evolution of predator-prey interactions: theory and evidence". Annual Review of Ecology and Systematics. 31: 79–105. doi:10.1146/annurev.ecolsys.31.1.79.
- Grimaldi, David; Engel, Michael S. (2005). Evolution of the Insects. Cambridge University Press. pp. 155–160. ISBN 978-0-521-82149-0.
- Grant, S. W. F.; Knoll, A. H.; Germs, G. J. B. (1991). "Probable Calcified Metaphytes in the Latest Proterozoic Nama Group, Namibia: Origin, Diagenesis, and Implications". Journal of Paleontology. 65 (1): 1–18. doi:10.1017/S002233600002014X. JSTOR 1305691. PMID 11538648.
- Awramik, S. M. (19 November 1971). "Precambrian columnar stromatolite diversity: Reflection of metazoan appearance". Science. 174 (4011): 825–827. Bibcode:1971Sci...174..825A. doi:10.1126/science.174.4011.825. PMID 17759393.
- Stanley, Steven M. (2008). "Predation defeats competition on the seafloor". Paleobiology. 34 (1): 1–21. doi:10.1666/07026.1.
- Loron, Corentin C.; Rainbird, Robert H.; Turner, Elizabeth C.; Wilder Greenman, J.; Javaux, Emmanuelle J. (2018). "Implications of selective predation on the macroevolution of eukaryotes: Evidence from Arctic Canada". Emerging Topics in Life Sciences. 2 (2): 247–255. doi:10.1042/ETLS20170153.
- Kelley, Patricia (2003). Predator--Prey Interactions in the Fossil Record. Springer. pp. 113–139, 141–176 and passim. ISBN 978-1-4615-0161-9. OCLC 840283264.
- Daley, Allison C. (2013). "Anomalocaridids". Current Biology. 23 (19): R860–R861. doi:10.1016/j.cub.2013.07.008. PMID 24112975.
- Anderson, P. S. L.; Westneat, M. (2009). "A biomechanical model of feeding kinematics for Dunkleosteus terrelli (Arthrodira, Placodermi)". Paleobiology. 35 (2): 251–269. doi:10.1666/08011.1.
- Carr, Robert K. (2010). "Paleoecology of Dunkleosteus terrelli (Placodermi: Arthrodira)". Kirtlandia. 57.
- Switeck, Brian (13 April 2012). "When Tyrannosaurus Chomped Sauropods". Journal of Vertebrate Paleontology. 25 (2): 469–472. doi:10.1671/0272-4634(2005)025[0469:TRFTUC]2.0.CO;2. Retrieved 24 August 2013.
- Darimont, C. T.; Fox, C. H.; Bryan, H. M.; Reimchen, T. E. (20 August 2015). "The unique ecology of human predators". Science. 349 (6250): 858–860. Bibcode:2015Sci...349..858D. doi:10.1126/science.aac4249. PMID 26293961.
- Gabriel, Otto; von Brandt, Andres (2005). Fish catching methods of the world. Blackwell. ISBN 978-0-85238-280-6.
- Griffin, Emma (2008). Blood Sport: Hunting in Britain Since 1066. Yale University Press. ISBN 978-0300145458.
- King, Richard J. (1 October 2013). The Devil's Cormorant: A Natural History. University of New Hampshire Press. p. 9. ISBN 978-1-61168-225-0.
- Glasier, Phillip (1998). Falconry and Hawking. Batsford. ISBN 978-0713484076.
- Aegerter, James; Fouracre, David; Smith, Graham C. (2017). Olsson, I Anna S (ed.). "A first estimate of the structure and density of the populations of pet cats and dogs across Great Britain". PLOS One. 12 (4): e0174709. doi:10.1371/journal.pone.0174709. PMC 5389805. PMID 28403172.
- The Humane Society of the United States. "U.S. Pet Ownership Statistics". Retrieved 27 April 2012.
- Liebenberg, Louis (2008). "The relevance of persistence hunting to human evolution". Journal of Human Evolution. 55 (6): 1156–1159. doi:10.1016/j.jhevol.2008.07.004. PMID 18760825.
- "Food For Thought" (PDF). The Life of Mammals. British Broadcasting Corporation. 31 October 2002.
- Flint, Maria Louise; Dreistadt, Steve H. (1998). Clark, Jack K. (ed.). Natural Enemies Handbook: The Illustrated Guide to Biological Pest Control. University of California Press. ISBN 978-0-520-21801-7.
- Johnston, Keith M. (2013). Science Fiction Film: A Critical Introduction. Berg Publishers. p. 98. ISBN 9780857850560.
- Newby, Richard (13 May 2018). "Is 'Predator' Finally Getting a Worthy Sequel?". Hollywood Reporter. Retrieved 7 September 2018.
- Schatz, Thomas. "The New Hollywood". Movie Blockbusters. p. 25. In: Stringer, Julian (2003). Movie Blockbusters. Routledge. pp. 15–44. ISBN 978-0-415-25608-7.
- Davison, Peter (1 December 2002). "Predators and Prey | Selected Poems, 1957-1994 by Ted Hughes". The New York Times. Retrieved 5 October 2018.
Hughes's earliest books contained a bewildering profusion of poems between their covers: ... fish and fowl, beasts of the field and forest, vigorous embodiments of predators and prey. Hughes as a student had taken up anthropology, not literature, and he chose to meditate his way into trancelike states of preconsciousness before committing poems to paper. His poems, early or late, enter into the relations of living creatures; they move in close to animal consciousness: The Thought-Fox, Esther's Tomcat, Pike.
- Gould, Stephen Jay (1995). The Tooth and Claw Centennial. Dinosaur in a Haystack. Harmony Books. pp. 63–75. ISBN 978-0517703939.
- Wallner, Astrid (18 July 2005). "The role of predators in Mythology". WaldWissen Information for Forest Management. Retrieved 5 October 2018. translated from Wallner, A. (1998) Die Bedeutung der Raubtiere in der Mythologie: Ergebnisse einer Literaturstudie. - Inf.bl. Forsch.bereiches Landsch.ökol. 39: 4-5.
- Kellert, Stephen R.; Black, Matthew; Rush, Colleen Reid; Bath, Alistair J. (1996). "Human Culture and Large Carnivore Conservation in North America". Conservation Biology. 10 (4): 977–990. doi:10.1046/j.1523-1739.1996.10040977.x.
Sources
- Barbosa, P.; Castellanos, I., eds. (2004). Ecology of predator-prey interactions. Oxford University Press. ISBN 978-0-19-517120-4.
- Beauchamp, Guy (2012). Social predation : how group living benefits predators and prey. Elsevier. ISBN 9780124076549.
- Bell, W. J. (2012). Searching Behaviour : the behavioural ecology of finding resources. Springer Netherlands. ISBN 9789401130981.
- Caro, Tim (2005). Antipredator Defenses in Birds and Mammals. University of Chicago Press. ISBN 978-0-226-09436-6.
- Cott, Hugh B. (1940). Adaptive Coloration in Animals. Methuen.
- Curio, E. (1976). The ethology of predation. Springer-Verlag. ISBN 978-0-387-07720-8.
- Jacobs, David Steve; Bastian, Anna (2017). Predator-prey interactions : co-evolution between bats and their prey. Springer. ISBN 9783319324920.
- Rockwood, Larry L. (2009). Introduction to population ecology. John Wiley & Sons. p. 281. ISBN 9781444309102.
- Ruxton, Graeme D.; Sherratt, Tom N.; Speed, Michael P. (2004). Avoiding attack: the evolutionary ecology of crypsis, warning signals, and mimicry. Oxford University Press. ISBN 9780198528593.|