Phenylboronic acid
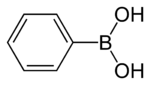
Phenylboronic acid or benzeneboronic acid, abbreviated as PhB(OH)2 where Ph is the phenyl group C6H5-, is a boronic acid containing a phenyl substituent and two hydroxyl groups attached to boron. Phenylboronic acid is white powder and is commonly used in organic synthesis. Boronic acids are mild Lewis acids which are generally stable and easy to handle, making them important to organic synthesis.
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Phenylboronic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.002.456 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H7BO2 | |
| Molar mass | 121.93 g/mol |
| Appearance | white to yellow powder |
| Odor | odorless |
| Melting point | 216 °C (421 °F; 489 K) |
| 10 g/L (20 ºC)[1] | |
| Solubility | soluble in diethyl ether, ethanol |
| Acidity (pKa) | 8.83 |
| Structure | |
| planar | |
| Thermochemistry | |
Std enthalpy of formation (ΔfH⦵298) |
-719.6 kJ/mol |
| Hazards | |
| Safety data sheet | |
| R-phrases (outdated) | 22 |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
740 mg/ml (rat, oral) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Properties
Phenylboronic acid is soluble in most polar organic solvents and is poorly soluble in hexanes and carbon tetrachloride. This planar compound has idealized C2V molecular symmetry. The boron atom is sp2-hybridized and contains an empty p-orbital. The orthorhombic crystals use hydrogen bonding to form units made up of two molecules.[2] These dimeric units are combined to give an extended hydrogen-bonded network. The molecule is planar with a minor bend around the C-B bond of 6.6° and 21.4° for the two PhB(OH)2 molecules.[3]
Synthesis
Numerous methods exist to synthesize phenylboronic acid. One of the most common synthesis uses phenylmagnesium bromide and trimethyl borate to form the ester PhB(OMe)2, which is then hydrolyzed to the product.[4]
- PhMgBr + B(OMe)3 → PhB(OMe)2 + MeOMgBr
- PhB(OMe)2 + H2O → PhB(OH)2 + MeOH
Other routes to phenylboronic acid involve Electrophilic borates to trap phenylmetal intermediates from phenyl halides or from directed ortho-metalation.[3] Phenylsilanes and phenylstannanes transmetalate with BBr3, followed by hydrolysis form phenylboronic acid. Aryl halides or triflates can be coupled with diboronyl reagents using transition metal catalysts. Aromatic C-H functionalization can also be done using transition metal catalysts.
Reactions
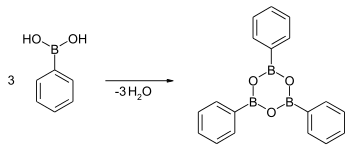
The dehydration of boronic acids gives boroxines, the trimeric anhydrides of phenylboronic acid. The dehydration reaction is driven thermally, sometimes with a dehydration agent.[5]
Phenylboronic acid participates in numerous cross coupling reactions where is serves as a source of a phenyl group. One example is the Suzuki reaction where, in the presence of a Pd(0) catalyst and base, phenylboronic acid and vinyl halides are coupled to produce phenyl alkenes.[6] This method was generalized to a route producing biaryls by coupling phenylboronic acid with aryl halides.
C-C bond forming processes commonly use phenylboronic acid as a reagent. Alpha-amino acids can be generated using the uncatalyzed reaction between alpha-ketoacids, amines, and phenylboronic acid.[7] Heck-type cross coupling of phenylboronic acid and alkenes and alkynes has been demonstrated.[8]
Aryl azides and nitroaromatics can also be generated using phenylboronic acid.[3] Phenylboronic acid can also be regioselectively halodeboronated using aqueous bromine, chlorine, or iodine:[9]
- PhB(OH)2 + Br2 + H2O → PhBr + B(OH)3 + HBr
Boronic esters result from the condensation of boronic acids with alcohols. This transformation is simply the replacement of the hydroxyl group by alkoxy or aryloxy groups.[3] This reversible reaction is commonly driven to product by the use of Dean-Stark apparatus or a dehydration agent to remove water.
- PhB(OH)2 + 2 ROH ⇌ PhB(OR)2 + 2 H2O
As an extension of this reactivity, PhB(OH)2 can be used as a protecting group for diols and diamines. This reactivity is the basis of the use of phenylboronic acid's use as a receptor and sensor for carbohydrates, antimicrobial agents, and enzyme inhibitors, neutron capture therapy for cancer, transmembrane transport, and bioconjugation and labeling of proteins and cell surface.[3]
See also
References
- http://m.chemicalbook.com/ChemicalProductProperty_EN_CB5323625.htm
- Rettig SJ, Trotter J (1977). "Crystal and molecular structure of phenylboronic acid, C6H5B(OH)2". Can. J. Chem. 55: 3071–3075. doi:10.1139/v77-430.
- Hall, D. G. Boronic Acids; WILEY-VCH: Edmonton, Canada, 2005. ISBN 3-527-30991-8
- Washburn, RM; Levens, E; Albright, CF; Billig, FA (1963). "Benzeneboronic anhydride". Organic Syntheses.; Collective Volume, 4, p. 68
- Snyder, H. R.; Kuck, J. A.; Johnson, J. R. (1938). "Organoboron Compounds, and the Study of Reaction Mechanisms. Primary Aliphatic Boronic Acids". J. Am. Chem. Soc. 60: 105. doi:10.1021/ja01268a033.
- Miyaura, N.; Suzuki, A. (1979). "Stereoselective synthesis of arylated (E)-alkenes by the reaction of alk-1-enylboranes with aryl halides in the presence of palladium catalyst". J. Chem. Soc., Chem. Commun. (19): 866. doi:10.1039/C39790000866.
- Petasis, N. A.; Xavialov, I. A. (1997). "A New and Practical Synthesis of α-Amino Acids from Alkenyl Boronic Acids". J. Am. Chem. Soc. 119 (2): 445. doi:10.1021/ja963178n.
- Sakai, M.; Hayashi, H.; Miyaura, N. (1998). "Rhodium-Catalyzed Addition of Organoboronic Acids to Aldehydes". Angew. Chem. Int. Ed. 37 (23): 3279. doi:10.1002/(SICI)1521-3773(19981217)37:23<3279::AID-ANIE3279>3.0.CO;2-M.
- Ainley, A. D.; Challenger, F. (1930). "Studies of the boron–carbon linkage. Part I. The oxidation and nitration of phenylboric acid". J. Chem. Soc.: 2171. doi:10.1039/JR9300002171.
Further reading
- Brown, H.C. Organic Synthesis via Boranses, Wiley, New York, 1975.
- Matteson, D. S. Stereodirected Synthesis with Organoboranes, Springer, Berlin, 1995. ISBN 978-3-540-59182-5
- Lappert, M. F. (1956). "Organic Compounds Of Boron". Chem. Rev. 56 (5): 959. doi:10.1021/cr50011a002.
- Pelter, A.; Smith, K.; Brown, H. C. Borane Reagents, Academic Press, New York, 1988.
- Mikhailov, B. M.; Bubnov, Y. N. Organoboron Compounds in Organic Synthesis, Harwood Academics, Glasgow, 1984. ISBN 3-7186-0113-3