Vinylogy
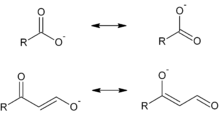
Vinylogy is the transmission of electronic effects through a conjugated organic bonding system.[1] The concept was introduced in 1926 by Ludwig Claisen to explain the acidic properties of formylacetone and related ketoaldehydes.[2] Its adjectival form, vinylogous, is used to describe functional groups in which the standard moieties of the group are separated only by a conjugated bonded system. For example, a carbon-carbon double bond (>C=C<, a "vinyl" moiety) between a carbonyl group and a hydroxyl group is referred to as a vinylogous carboxylic acid, analogous electronically to a carboxylic acid, RCOOH.
Vinylogous reactivity
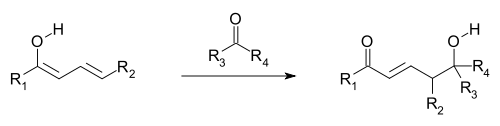
Vinylogous reactions are believed to occur when orbitals of the double bonds of the vinyl group and of an attached electron-withdrawing group (EWG; the π orbitals) are aligned and so can overlap and mix (i.e., are conjugated). Electron delocalization enables the EWG to receive electron density through participation of the conjugated system. Vinylogous reactions also include conjugate additions, where a nucleophile reacts at the vinyl terminus, as well as a vinylogous variation of the aldol reaction, where an electrophile is attacked by a nucleophilic vinylogous enolate (see first and following image). The vinylogous enolate reacts at the terminal position of the double bond system (the γ-carbon), rather than the α-carbon immediately adjacent to the carbonyl, as would a simple enolate. Allylic electrophiles often react by vinylogous attack of a nucleophile rather than direct addition.
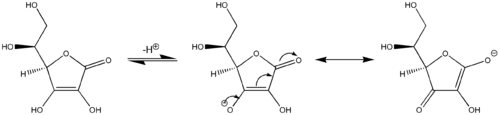
A further example of vinylogous reactivity: ascorbic acid (Vitamin C) behaves as a vinylogous carboxylic acid by involvement of its carbonyl moiety, a vinyl group within the ring, and the lone pair on the hydroxyl group acting as the conjugated system. Acidity of the hydroxyl proton at the terminus of the vinyl group in ascorbic acid is more comparable to a typical carboxylic acid than an alcohol because two major resonance structures stabilize the negative charge on the conjugate base of ascorbic acid (center and right structures in last image), analogous to the two resonance structures that stabilize the negative charge on the anion that results from removal of a proton from a simple carboxylic acid (cf. first image).
Further reading
- Lisboa, Marilda P.; Hoang, Tung T.; Dudley, Gregory B. (2011). "Tandem Nucleophilic Addition / Fragmentation of Vinylogous Acyl Triflates: 2-Methyl-2-(1-Oxo-5-Heptynyl)-1,3-Dithiane". Organic Syntheses. 88: 353. doi:10.15227/orgsyn.088.0353.
References
- The Vinylogous Aldol Reaction: A Valuable, Yet Understated Carbon-Carbon Bond-Forming Maneuver Giovanni Casiraghi, Franca Zanardi, Giovanni Appendino, and Gloria Rassu Chem. Rev. 2000; 100(6) pp 1929 - 1972; (Review) doi:10.1021/cr990247i
- Zu den O-Alkylderivaten des Benzoyl-acetons und den aus ihnen entstehenden Isoxazolen. (Entgegnung an Hrn. O. Weygand.) Berichte der deutschen chemischen Gesellschaft (A and B Series) Volume 59, Issue 2, Date: 10. February 1926, Pages: 144-153 L. Claisen. doi:10.1002/cber.19260590206