Vardenafil
Vardenafil is a PDE5 inhibitor used for treating erectile dysfunction that is sold under the trade names Levitra, Staxyn, and Vivanza.
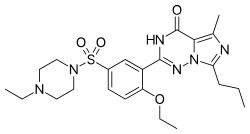 | |
| Clinical data | |
|---|---|
| Trade names | Levitra, Staxyn, Vivanza |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a603035 |
| License data | |
| Pregnancy category | |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 15% |
| Protein binding | 95% |
| Metabolism | Hepatic (CYP3A4) |
| Elimination half-life | 4–5 hours |
| Excretion | Biliary |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.112.672 |
| Chemical and physical data | |
| Formula | C23H32N6O4S |
| Molar mass | 488.61 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Medical use
Vardenafil's indications and contraindications are the same as with other PDE5 inhibitors; it is closely related in function to sildenafil citrate (Viagra) and tadalafil (Cialis). The difference between the vardenafil molecule and sildenafil citrate is a nitrogen atom's position and the change of sildenafil's piperazine ring methyl group to an ethyl group. Tadalafil is structurally different from both sildenafil and vardenafil. Vardenafil's relatively short effective time is comparable to but somewhat longer than sildenafil's.
Beyond its indications for erectile dysfunction, vardenafil may be effective in the treatment of premature ejaculation, where it may significantly increase the time from penetration to ejaculation.[1]
Adverse reactions
The common, adverse drug reactions (side effects) are the same as with other PDE5 inhibitors. The frequent vardenafil-specific side-effect is nausea; the infrequent side effects are abdominal pain, back pain, photosensitivity, abnormal vision, eye pain, facial edema, hypotension, palpitation, tachycardia, arthralgia, myalgia, rash, itch, and priapism.
One possibly serious, but rare, side effect with vardenafil is heart attack. Also, in rare cases, vardenafil use may cause priapism, a very painful emergency condition that can cause impotence if left untreated.[2]
On 18 October 2007, the U.S. Food and Drug Administration (FDA) announced that a warning about possible deafness (sudden hearing loss) would be added to the drug labels of vardenafil, and other PDE5 inhibitors.[3]
Interactions
Vardenafil, as with all PDE5 inhibitors, should not be used by people taking nitrate medications, because combining them with vardenafil might provoke potentially life-threatening hypotension (low blood pressure).[4][5][6]
Further, vardenafil causes lengthening of the QT interval. Therefore, it should not be taken by people taking other medications that affect the QT interval (such as amiodarone).[7][8]
History
Vardenafil was co-marketed by Bayer Pharmaceuticals, GlaxoSmithKline, and Schering-Plough under the trade name Levitra. As of 2005, the co-promotion rights of GSK on Levitra have been returned to Bayer in many markets outside the U.S. In Italy, Bayer sells vardenafil as Levitra and GSK sells it as Vivanza. Thus, because of European Union trade rules, parallel imports might result in Vivanza sold next to Levitra in the EU.
An orally disintegrating form, marketed as Staxyn and Levitra Soft, has been gaining approvals in countries such as the United States[9] and Canada.[10]
Dose forms

It is available in 2.5 mg, 5 mg, 10 mg, and 20 mg doses in round orange tablets. The normal starting dose is 10 mg[11] (roughly equivalent to 50 mg of sildenafil). Vardenafil should be taken 1 to 2 hours prior to sexual activity, with a maximum dose frequency of once per day. In some territories, such as the UK, only certain doses may be available.
Vardenafil is also available under the name Staxyn as a tablet which dissolves on the tongue rather than being swallowed in the form of a pill.[12]
Tainted supplements
The USFDA has found vardenafil and other synthetic PDE5 inhibitors in numerous products marketed as "herbal" supplements or "all natural" products for male enhancement.[13][14]
Notes
- Aversa A, Pili M, Francomano D, Bruzziches R, Spera E, La Pera G, Spera G (July 2009). "Effects of vardenafil administration on intravaginal ejaculatory latency time in men with lifelong premature ejaculation". International Journal of Impotence Research. 21 (4): 221–7. doi:10.1038/ijir.2009.21. PMID 19474796.
- Schools of Pharmacy (Glen L. Stimmel, Pharm.D., and Mary A. Gutierrez, Pharm.D.) and Medicine (Glen L. Stimmel, Pharm.D.), University of Southern California, Los Angeles, California. "Counseling Patients About Sexual Issues: Drug-Induced Priapism". Medscape. Retrieved 2010-12-06.CS1 maint: multiple names: authors list (link)
- "FDA Announces Revisions to Labels for Cialis, Levitra and Viagra". Food and Drug Administration. 2007-10-18. Retrieved 2009-08-06.
- Kloner RA (December 2005). "Pharmacology and drug interaction effects of the phosphodiesterase 5 inhibitors: focus on alpha-blocker interactions". The American Journal of Cardiology. 96 (12B): 42M–46M. doi:10.1016/j.amjcard.2005.07.011. PMID 16387566.
- Carson CC (February 2006). "PDE5 inhibitors: are there differences?". The Canadian Journal of Urology. 13 Suppl 1: 34–9. PMID 16526979.
- "Levitra for Treatment of Erectile Dysfunction". www.erectiledysfunction.com. Retrieved 2017-10-27.
- Lepor H, Lepor NE, Hill LA, Trohman RG (2008). "The QT Interval and Selection of Alpha-Blockers for Benign Prostatic Hyperplasia". Reviews in Urology. 10 (2): 85–91. PMC 2483321. PMID 18660858.
- Learning, Jones & Bartlett (2010-06-29). 2011 Nurse's Drug Handbook. Jones & Bartlett Publishers. ISBN 9781449653729.
- "New erectile dysfunction treatment STAXYN™ approved in the U.S. - Pharmaceutical Processing". pharmpro.com. 2010-06-21. Archived from the original on 2012-04-06.
- "STAXYN® - New Innovation in Erectile Dysfunction Helps Younger Men Rise to the Occasion".
- "Levitra Dosage". Drugs.com. Retrieved 2018-02-23.
- "Vardenafil". Medicine Net. Retrieved 2014-02-18.
- "Tainted Products Marketed as Dietary Supplements". United States Food and Drug Administration.
- Tucker J, Fischer T, Upjohn L, Mazzera D, Kumar M (October 2018). "Unapproved Pharmaceutical Ingredients Included in Dietary Supplements Associated With US Food and Drug Administration Warnings". JAMA Network Open. 1 (6): e183337. doi:10.1001/jamanetworkopen.2018.3337. PMC 6324457. PMID 30646238.
External links
- "Vardenafil". Drug Information Portal. U.S. National Library of Medicine.