Oxindole
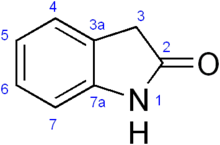
Oxindole (2-indolone) is an aromatic heterocyclic organic compound. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered nitrogen-containing ring. Oxindole is a modified indoline with a substituted carbonyl at the second position of the 5-member indoline ring.
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Oxindole | |
| Systematic IUPAC name
1H-indol-2(3H)-one | |
| Identifiers | |
3D model (JSmol) |
|
| 114692 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.390 |
| EC Number |
|
| 637057 | |
| KEGG | |
| MeSH | Oxindole |
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C8H7NO | |
| Molar mass | 133.150 g·mol−1 |
| Melting point | 128 °C (262 °F; 401 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Oxindole is a tryptophan derivative and in human biology is formed by gut bacteria ("normal flora"). It is normally metabolized and detoxified from the body by the liver. In excess, it can cause sedation, muscle weakness, hypotension, and coma. Patients with hepatic encephalopathy have been recorded to have elevated serum oxindole levels.[1]
Related aromatic compounds
- Indoline
- Indole
- Indene
- Benzofuran
- Isoindoline
- Carboline
- Isatin
- Methylindole
- Carbazole
- Pyrrole
- Skatole
- Benzene
gollark: In the meantime you can… probably use an oven, microwave or macrowave somewhere else?
gollark: Just buy your own electrons. Become independence or something.
gollark: Er, check out a JS library called regl?
gollark: WebGL.
gollark: ?
References
- Riggio, Oliviero; Mannaioni, Guido; Ridola, Lorenzo; Angeloni, Stefania; Merli, Manuela; Carlà, Vincenzo; Salvatori, Filippo Maria; Moroni, Flavio (2 February 2010). "Peripheral and Splanchnic Indole and Oxindole Levels in Cirrhotic Patients: A Study on the Pathophysiology of Hepatic Encephalopathy". The American Journal of Gastroenterology. 105 (6): 1374–1381. doi:10.1038/ajg.2009.738. PMID 20125128.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.