Polystyrene
Polystyrene (PS) /ˌpɒliˈstaɪriːn/ is a synthetic aromatic hydrocarbon polymer made from the monomer known as styrene.[5] Polystyrene can be solid or foamed. General-purpose polystyrene is clear, hard, and rather brittle. It is an inexpensive resin per unit weight. It is a rather poor barrier to oxygen and water vapour and has a relatively low melting point.[6] Polystyrene is one of the most widely used plastics, the scale of its production being several million tonnes per year.[7] Polystyrene can be naturally transparent, but can be coloured with colourants. Uses include protective packaging (such as packing peanuts and CD and DVD cases), containers, lids, bottles, trays, tumblers, disposable cutlery[6] and in the making of models.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Poly(1-phenylethene) | |
| Other names
Thermocol | |
| Identifiers | |
| Abbreviations | PS |
| ChemSpider |
|
| ECHA InfoCard | 100.105.519 |
CompTox Dashboard (EPA) |
|
| Properties | |
| (C8H8)n | |
| Density | 0.96–1.05 g/cm3 |
| Melting point | ~ 240 °C (464 °F; 513 K)[1] For Isotactic Polystyrene |
| Boiling point | 430 °C (806 °F; 703 K) and depolymerizes |
| Insoluble | |
| Solubility | Soluble in benzene, carbon disulfide, chlorinated aliphatic hydrocarbons, chloroform, cyclohexanone, dioxane, ethyl acetate, ethylbenzene, MEK, NMP, THF [2] |
| Thermal conductivity | 0.033 W/(m·K) (foam, ρ 0.05 g/cm3)[3] |
Refractive index (nD) |
1.6; dielectric constant 2.6 (1 kHz – 1 GHz)[4] |
| Related compounds | |
Related compounds |
Styrene (monomer) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |



As a thermoplastic polymer, polystyrene is in a solid (glassy) state at room temperature but flows if heated above about 100 °C, its glass transition temperature. It becomes rigid again when cooled. This temperature behaviour is exploited for extrusion (as in Styrofoam) and also for molding and vacuum forming, since it can be cast into molds with fine detail.
Under ASTM standards, polystyrene is regarded as not biodegradable. It is accumulating as a form of litter in the outside environment, particularly along shores and waterways, especially in its foam form, and in the Pacific Ocean.[8]
History
Polystyrene was discovered in 1839 by Eduard Simon, an apothecary from Berlin.[9] From storax, the resin of the Oriental sweetgum tree Liquidambar orientalis, he distilled an oily substance, a monomer that he named styrol. Several days later, Simon found that the styrol had thickened into a jelly he dubbed styrol oxide ("Styroloxyd") because he presumed an oxidation. By 1845 Jamaican-born chemist John Buddle Blyth and German chemist August Wilhelm von Hofmann showed that the same transformation of styrol took place in the absence of oxygen.[10] They called the product "meta styrol"; analysis showed that it was chemically identical to Simon's Styroloxyd.[11] In 1866 Marcellin Berthelot correctly identified the formation of meta styrol/Styroloxyd from styrol as a polymerisation process.[12] About 80 years later it was realized that heating of styrol starts a chain reaction that produces macromolecules, following the thesis of German organic chemist Hermann Staudinger (1881–1965). This eventually led to the substance receiving its present name, polystyrene.
The company I. G. Farben began manufacturing polystyrene in Ludwigshafen, about 1931, hoping it would be a suitable replacement for die-cast zinc in many applications. Success was achieved when they developed a reactor vessel that extruded polystyrene through a heated tube and cutter, producing polystyrene in pellet form.
Otis Ray McIntire (1918-1996) a chemical engineer of Dow Chemical rediscovered a process first patented by Swedish inventor Carl Munters.[13] According to the Science History Institute, "Dow bought the rights to Munters’s method and began producing a lightweight, water-resistant, and buoyant material that seemed perfectly suited for building docks and watercraft and for insulating homes, offices, and chicken sheds."[14] In 1944, Styrofoam was patented.
Before 1949, chemical engineer Fritz Stastny (1908–1985) developed pre-expanded PS beads by incorporating aliphatic hydrocarbons, such as pentane. These beads are the raw material for molding parts or extruding sheets. BASF and Stastny applied for a patent that was issued in 1949. The molding process was demonstrated at the Kunststoff Messe 1952 in Düsseldorf. Products were named Styropor.
The crystal structure of isotactic polystyrene was reported by Giulio Natta.[15]
In 1954, the Koppers Company in Pittsburgh, Pennsylvania, developed expanded polystyrene (EPS) foam under the trade name Dylite.[16] In 1960, Dart Container, the largest manufacturer of foam cups, shipped their first order.[17]
Structure
In chemical terms, polystyrene is a long chain hydrocarbon wherein alternating carbon centers are attached to phenyl groups (a derivative of benzene). Polystyrene's chemical formula is (C
8H
8)
n; it contains the chemical elements carbon and hydrogen.
The material's properties are determined by short-range van der Waals attractions between polymers chains. Since the molecules consist of thousands of atoms, the cumulative attractive force between the molecules is large. When heated (or deformed at a rapid rate, due to a combination of viscoelastic and thermal insulation properties), the chains can take on a higher degree of confirmation and slide past each other. This intermolecular weakness (versus the high intramolecular strength due to the hydrocarbon backbone) confers flexibility and elasticity. The ability of the system to be readily deformed above its glass transition temperature allows polystyrene (and thermoplastic polymers in general) to be readily softened and molded upon heating. Extruded polystyrene is about as strong as an unalloyed aluminium but much more flexible and much less dense (1.05 g/cm3 for polystyrene vs. 2.70 g/cm3 for aluminium).
Production
Polystyrene is an addition polymer that results when styrene monomers interconnect (polymerization). In the polymerization, the carbon-carbon π bond of the vinyl group is broken and a new carbon-carbon σ bond is formed, attaching to the carbon of another styrene monomer to the chain. Since only one kind of monomer is used in its preparation, it is a homopolymer. The newly formed σ bond is stronger than the π bond that was broken, thus it is difficult to depolymerize polystyrene. About a few thousand monomers typically comprise a chain of polystyrene, giving a molecular weight of 100,000–400,000 g/mol.
Each carbon of the backbone has tetrahedral geometry, and those carbons that have a phenyl group (benzene ring) attached are stereogenic. If the backbone were to be laid as a flat elongated zig-zag chain, each phenyl group would be tilted forward or backward compared to the plane of the chain.
The relative stereochemical relationship of consecutive phenyl groups determines the tacticity, which affects various physical properties of the material.
Tacticity
In polystyrene, tacticity describes the extent to which the phenyl group is uniformly aligned (arranged at one side) in the polymer chain. Tacticity has a strong effect on the properties of the plastic. Standard polystyrene is atactic. The diastereomer where all of the phenyl groups are on the same side is called isotactic polystyrene, which is not produced commercially.
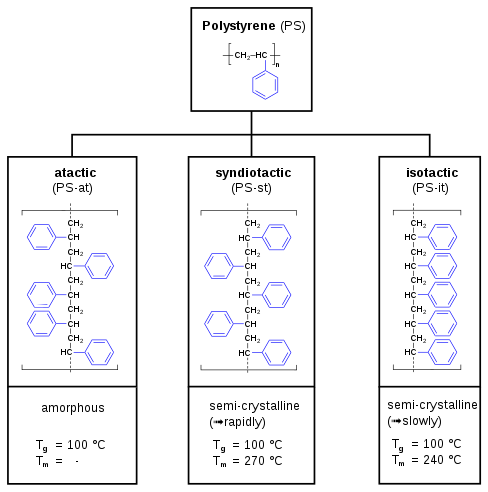
Atactic polystyrene
The only commercially important form of polystyrene is atactic, in which the phenyl groups are randomly distributed on both sides of the polymer chain. This random positioning prevents the chains from aligning with sufficient regularity to achieve any crystallinity. The plastic has a glass transition temperature Tg of ~90 °C. Polymerization is initiated with free radicals.[7]
Syndiotactic polystyrene
Ziegler–Natta polymerization can produce an ordered syndiotactic polystyrene with the phenyl groups positioned on alternating sides of the hydrocarbon backbone. This form is highly crystalline with a Tm of 270 °C (518 °F). Syndiotactic polystyrene resin is currently produced under the trade name XAREC by Idemitsu corporation, who use a metallocene catalyst for the polymerisation reaction.[18]
Degradation
Polystyrene is relatively chemically inert. While it is waterproof and resistant to breakdown by many acids and bases, it is easily attacked by many organic solvents (e.g. it dissolves quickly when exposed to acetone), chlorinated solvents, and aromatic hydrocarbon solvents. Because of its resilience and inertness, it is used for fabricating many objects of commerce. Like other organic compounds, polystyrene burns to give carbon dioxide and water vapor, in addition to other thermal degradation by-products. Polystyrene, being an aromatic hydrocarbon, typically combusts incompletely as indicated by the sooty flame.
The process of depolymerizing polystyrene into its monomer, styrene, is called pyrolysis. This involves using high heat and pressure to break down the chemical bonds between each styrene compound. Pyrolysis usually goes up to 430 °C.[19] The high energy cost of doing this has made commercial recycling of polystyrene back into styrene monomer difficult.
Organisms
Polystyrene is generally considered to be non-biodegradable. However, certain organisms are able to degrade it, albeit very slowly.[20]
In 2015, researchers discovered that mealworms, the larvae form of the darkling beetle Tenebrio molitor, could digest and subsist healthily on a diet of EPS.[21][22] About 100 mealworms could consume between 34 and 39 milligrams of this white foam in a day. The droppings of mealworm were found to be safe for use as soil for crops.[21]
In 2016, it was also reported that superworms (Zophobas morio) may eat expanded polystyrene (EPS).[23] A group of high school students in Ateneo de Manila University found that compared to Tenebrio molitor larvae, Zophobas morio larvae may consume greater amounts of EPS over longer periods of time.[24]
Pseudomonas putida is capable of converting styrene oil into the biodegradable plastic PHA.[25][26][27] This may someday be of use in the effective disposing of polystyrene foam. It is worthy to note the polystyrene must undergo pyrolysis to turn into styrene oil.
Forms produced
| Properties | |
|---|---|
| Density of EPS | 16–640 kg/m3[28] |
| Young's modulus (E) | 3000–3600 MPa |
| Tensile strength (st) | 46–60 MPa |
| Elongation at break | 3–4% |
| Charpy impact test | 2–5 kJ/m2 |
| Glass transition temperature | 100 °C[29] |
| Vicat softening point | 90 °C[30] |
| Coefficient of thermal expansion | 8×10−5 /K |
| Specific heat capacity (c) | 1.3 kJ/(kg·K) |
| Water absorption (ASTM) | 0.03–0.1 |
| Decomposition | X years, still decaying |
Polystyrene is commonly injection molded, vacuum formed, or extruded, while expanded polystyrene is either extruded or molded in a special process. Polystyrene copolymers are also produced; these contain one or more other monomers in addition to styrene. In recent years the expanded polystyrene composites with cellulose[31][32] and starch[33] have also been produced. Polystyrene is used in some polymer-bonded explosives (PBX).
Sheet or molded polystyrene

Polystyrene (PS) is used for producing disposable plastic cutlery and dinnerware, CD "jewel" cases, smoke detector housings, license plate frames, plastic model assembly kits, and many other objects where a rigid, economical plastic is desired. Production methods include thermoforming (vacuum forming) and injection molding.
Polystyrene Petri dishes and other laboratory containers such as test tubes and microplates play an important role in biomedical research and science. For these uses, articles are almost always made by injection molding, and often sterilized post-molding, either by irradiation or by treatment with ethylene oxide. Post-mold surface modification, usually with oxygen-rich plasmas, is often done to introduce polar groups. Much of modern biomedical research relies on the use of such products; they, therefore, play a critical role in pharmaceutical research.[34]
Thin sheets of polystyrene are used in polystyrene film capacitors as it forms a very stable dielectric, but has largely fallen out of use in favor of polyester.
Foams
Polystyrene foams are 95-98% air.[35][36] Polystyrene foams are good thermal insulators and are therefore often used as building insulation materials, such as in insulating concrete forms and structural insulated panel building systems. Grey polystyrene foam, incorporating graphite has superior insulation properties.[37]
PS foams also exhibit good damping properties, therefore it is used widely in packaging. The trademark Styrofoam by Dow Chemical Company is informally used (mainly US & Canada) for all foamed polystyrene products, although strictly it should only be used for "extruded closed-cell" polystyrene foams made by Dow Chemicals.
Foams are also used for non-weight-bearing architectural structures (such as ornamental pillars).
Expanded polystyrene (EPS)

.jpg)
Expanded polystyrene (EPS) is a rigid and tough, closed-cell foam with a normal density range of 11 to 32 kg/m3.[38] It is usually white and made of pre-expanded polystyrene beads. EPS is used for food containers, molded sheets for building insulation, and packing material either as solid blocks formed to accommodate the item being protected or as loose-fill "peanuts" cushioning fragile items inside boxes. A significant portion of all EPS products are manufactured through injection molding. Mold tools tend to be manufactured from steels (which can be hardened and plated), and aluminum alloys. The molds are controlled through a split via a channel system of gates and runners.[39] EPS is colloquially called "styrofoam" in the United States and Canada, an incorrectly applied genericization of Dow Chemical's brand of extruded polystyrene.[40]
EPS in building construction
Sheets of EPS are commonly packaged as rigid panels (Common in Europe is a size of 100cmx50cm, usually depending on an intended type of connection and glue techniques, it is, in fact, 99.5cmx49.5 cm or 98cmx48cm; less common is 120x60cm; size 4 by 8 or 2 by 8 feet in the United States). Common thicknesses are from 10mm to 500mm. Many customizations, additives, and thin additional external layers on one or both sides are often added to help with various properties.
Thermal conductivity is measured according to EN 12667. Typical values range from 0.032 to 0.038 W/(m·K) depending on the density of the EPS board. The value of 0.038 W/(m·K) was obtained at 15 kg/m3 while the value of 0.032 W/(m·K) was obtained at 40 kg/m3 according to the datasheet of K-710 from StyroChem Finland. Adding fillers (graphites, aluminum, or carbons) has recently allowed the thermal conductivity of EPS to reach around 0.030–0.034 (as low as 0.029) and as such has a grey/black color which distinguishes it from standard EPS. Several EPS producers have produced a variety of these increased thermal resistance EPS usage for this product in the UK & EU.
Water vapor diffusion resistance (μ) of EPS is around 30–70.
ICC-ES (International Code Council Evaluation Service) requires EPS boards used in building construction meet ASTM C578 requirements. One of these requirements is that the limiting oxygen index of EPS as measured by ASTM D2863 be greater than 24 volume %. Typical EPS has an oxygen index of around 18 volume %; thus, a flame retardant is added to styrene or polystyrene during the formation of EPS.
The boards containing a flame retardant when tested in a tunnel using test method UL 723 or ASTM E84 will have a flame spread index of less than 25 and a smoke-developed index of less than 450. ICC-ES requires the use of a 15-minute thermal barrier when EPS boards are used inside of a building.
According to the EPS-IA ICF organization, the typical density of EPS used for insulated concrete forms (expanded polystyrene concrete) is 1.35 to 1.80 PCF. This is either Type II or Type IX EPS according to ASTM C578. EPS blocks or boards used in building construction are commonly cut using hot wires.[41]
Extruded polystyrene (XPS)
Extruded polystyrene foam (XPS) consists of closed cells. It offers improved surface roughness, higher stiffness and reduced thermal conductivity. The density range is about 28–45 kg/m3.
Extruded polystyrene material is also used in crafts and model building, in particular architectural models. Because of the extrusion manufacturing process, XPS does not require facers to maintain its thermal or physical property performance. Thus, it makes a more uniform substitute for corrugated cardboard. Thermal conductivity varies between 0.029 and 0.039 W/(m·K) depending on bearing strength/density and the average value is ~0.035 W/(m·K).
Water vapor diffusion resistance (μ) of XPS is around 80–250.
Commonly extruded polystyrene foam materials include:
- Styrofoam, also known as Blue Board, produced by Dow Chemical Company
- Depron, a thin insulation sheet also used for model building[42]
Water absorption of polystyrene foams
Although it is a closed-cell foam, both expanded and extruded polystyrene are not entirely waterproof or vapor proof.[43] In expanded polystyrene there are interstitial gaps between the expanded closed-cell pellets that form an open network of channels between the bonded pellets, and this network of gaps can become filled with liquid water. If the water freezes into ice, it expands and can cause polystyrene pellets to break off from the foam. Extruded polystyrene is also permeable by water molecules and can not be considered a vapor barrier.[44]
Water-logging commonly occurs over a long period in polystyrene foams that are constantly exposed to high humidity or are continuously immersed in water, such as in hot tub covers, in floating docks, as supplemental flotation under boat seats, and for below-grade exterior building insulation constantly exposed to groundwater.[45] Typically an exterior vapor barrier such as impermeable plastic sheeting or a sprayed-on coating is necessary to prevent saturation.
Oriented polystyrene
Oriented polystyrene (OPS) is produced by stretching extruded PS film, improving visibility through the material by reducing haziness and increasing stiffness. This is often used in packaging where the manufacturer would like the consumer to see the enclosed product. Some benefits to OPS are that it is less expensive to produce than other clear plastics such as polypropylene (PP), (PET), and high-impact polystyrene (HIPS), and it is less hazy than HIPS or PP. The main disadvantage of OPS is that it is brittle, and will crack or tear easily.
Co-polymers
Ordinary (homo-polymeric) polystyrene has an excellent property profile about transparency, surface quality and stiffness. Its range of applications is further extended by co-polymerization and other modifications (blends e.g. with PC and syndiotactic polystyrene).[46]:102–104 Several copolymers are used based on styrene: The crispiness of homopolymeric polystyrene is overcome by elastomer-modified styrene-butadiene copolymers. Copolymers of styrene and acrylonitrile (SAN) are more resistant to thermal stress, heat and chemicals than homopolymers and are also transparent. Copolymers called ABS have similar properties and can be used at low temperatures, but they are opaque.
Styrene-butane co-polymers
Styrene-butane co-polymers can be produced with a low butene content. Styrene-butane co-polymers include PS-I and SBC (see below), both co-polymers are impact resistant. PS-I is prepared by graft co-polymerization, SBC by anionic block co-polymerization, which makes it transparent in case of appropriate block size.[47]
If styrene-butane co-polymer has a high butylene content, styrene-butadiene rubber (SBR) is formed.
The impact strength of styrene-butadiene co-polymers is based on phase separation, polystyrene and poly-butane are not soluble in each other (see Flory-Huggins theory). Co-polymerization creates a boundary layer without complete mixing. The butadiene fractions (the "rubber phase") assemble to form particles embedded in a polystyrene matrix. A decisive factor for the improved impact strength of styrene-butadiene copolymers is their higher absorption capacity for deformation work. Without applied force, the rubber phase initially behaves like a filler. Under tensile stress, crazes (microcracks) are formed, which spread to the rubber particles. The energy of the propagating crack is then transferred to the rubber particles along its path. A large number of cracks give the originally rigid material a laminated structure. The formation of each lamella contributes to the consumption of energy and thus to an increase in elongation at break. Polystyrene homo-polymers deform when a force is applied until they break. Styrene-butane co-polymers do not break at this point, but begin to flow, solidify to tensile strength and only break at much higher elongation.[48]:426
With a high proportion of polybutadiene, the effect of the two phases is reversed. Styrene-butadiene rubber behaves like an elastomer but can be processed like a thermoplastic.
Impact-resistant polystyrene (PS-I)
PS-I (impact resistant polystyrene) consists of a continuous polystyrene matrix and a rubber phase dispersed therein. It is produced by polymerization of styrene in the presence of polybutadiene dissolved (in styrene). Polymerization takes place simultaneously in two ways:[49]
- Graft copolymerization: The growing polystyrene chain reacts with a double bond of the polybutadiene. As a result, several polystyrene chains are attached to one polybutadiene molecule.
- Homopolymerization: Styrene polymerizes to polystyrene and does not react with the present polybutadiene.
The polybutadiene particles (rubber particles) in PS-I usually have a diameter of 0.5 - 9 μm. They thereby scatter visible light, making PS-I opaque.[50]:476 The material is stable (no further phase segregation occurs) because polybutadiene and polystyrene are chemically linked.[51] Historically, PS-I was first produced by simple mixing (physical mixing, called blending) of polybutadiene and polystyrene. This way, a polymer blend is produced, not a copolymer. However, the polyblend material has considerably worse properties.[50]:476
Styrene-butadiene block co-polymers
SBS (styrene-butadiene-styrene block copolymer) is made by anionic block copolymerization and consists of three blocks:[52]
SSSSSSSSSSSSSSSSSSSSBBBBBBBBBBBBBBBBBBBBSSSSSSSSSSSSSSSSSSSS
S represents in the figure the styrene repeat unit, B the butadiene repeat unit. However, the middle block often does not consist of such depicted butane homo-polymer but of a styrene-butadiene co-polymer:
SSSSSSSSSSSSSSSSSSSBBSBBSBSBBBBSBSSBBBSBSSSSSSSSSSSSSSSSSSSS
By using a statistical copolymer at this position, the polymer becomes less susceptible to cross-linking and flows better in the melt. For the production of SBS, the first styrene is homopolymerized via anionic copolymerization. Typically, an organometallic compound such as butyllithium is used as a catalyst. Butadiene is then added and after styrene again its polymerization. The catalyst remains active during the whole process (for which the used chemicals must be of high purity). The molecular weight distribution of the polymers is very low (polydispersity in the range of 1.05, the individual chains have thus very similar lengths). The length of the individual blocks can be adjusted by the ratio of catalyst to monomer. The size of the rubber sections, in turn, depends on the block length. The production of small structures (smaller than the wavelength of the light) ensure transparency. In contrast to PS-I, however, the block copolymer does not form any particles but has a lamellar structure.
Styrene-butadiene rubber
Styrene-butadiene rubber (SBR) is produced like PS-I by graft copolymerization, but with a lower styrene content. Styrene-butadiene rubber thus consists of a rubber matrix with a polystyrene phase dispersed therein.[51] Unlike PS-I and SBC, it is not a thermoplastic, but an elastomer. Within the rubber phase, the polystyrene phase is assembled into domains. This causes physical cross-linking on a microscopic level. When the material is heated above the glass transition point, the domains disintegrate, the cross-linking is temporarily suspended and the material can be processed like a thermoplastic.[53]
Acrylonitrile butadiene styrene
Acrylonitrile butadiene styrene (ABS) is a material that is stronger than pure polystyrene.
Others
SMA is a copolymer with maleic anhydride. Styrene can be copolymerized with other monomers; for example, divinylbenzene can be used for cross-linking the polystyrene chains to give the polymer used in solid phase peptide synthesis. Styrene-acrylonitrile resin (SAN) has a greater thermal resistance than pure styrene.
Environmental issues
Production
Polystyrene foams are produced using blowing agents that form bubbles and expand the foam. In expanded polystyrene, these are usually hydrocarbons such as pentane, which may pose a flammability hazard in manufacturing or storage of newly manufactured material, but have relatively mild environmental impact. Extruded polystyrene is usually made with hydro-fluorocarbons (HFC-134a),[54] which have global warming potentials of approximately 1000–1300 times that of carbon dioxide.[55]
Non-biodegradable
Waste polystyrene takes hundreds of years to biodegrade and is resistant to photo-oxidation.[56]
Litter
.jpg) Polystyrene waste
Polystyrene waste Coastal debris including polystyrene
Coastal debris including polystyrene
Animals do not recognize polystyrene foam as an artificial material and may even mistake it for food.[57] Polystyrene foam blows in the wind and floats on water, due to its low specific gravity. It can have serious effects on the health of birds or marine animals that swallow significant quantities.[57]
Reducing
Restricting the use of foamed polystyrene takeout food packaging is a priority of many solid waste environmental organisations.[58] Efforts have been made to find alternatives to polystyrene, especially foam in restaurant settings. The original impetus was to eliminate chlorofluorocarbons (CFC), which was a former component of foam.
United States
In 1987, Berkeley, California, banned CFC food containers.[59] The following year, Suffolk County, New York, became the first U.S. jurisdiction to ban polystyrene in general.[60] However, legal challenges by the Society of the Plastics Industry[61] kept the ban from going into effect until at last it was delayed when the Republican and Conservative parties gained the majority of the county legislature.[62] In the meantime, Berkeley became the first city to ban all foam food containers.[63] As of 2006, about one hundred localities in the United States, including Portland, Oregon, and San Francisco had some sort of ban on polystyrene foam in restaurants. For instance, in 2007 Oakland, California, required restaurants to switch to disposable food containers that would biodegrade if added to food compost.[64] In 2013, San Jose became reportedly the largest city in the country to ban polystyrene foam food containers.[65] Some communities have implemented wide polystyrene bans, such as Freeport, Maine, which did so in 1990.[66] In 1988, the first U.S. ban of general polystyrene foam was enacted in Berkeley, California.[63]
On July 1, 2015, New York City became the largest city in the United States to attempt to prohibit the sale, possession, and distribution of single-use polystyrene foam (the initial decision was overturned on appeal).[67] In San Francisco, supervisors approved the toughest ban on "Styrofoam" (EPS) in the US which went into effect January 1, 2017. The city's Department of the Environment can make exceptions for certain uses like shipping medicines at prescribed temperatures.[68]
The U.S. Green Restaurant Association does not allow polystyrene foam to be used as part of its certification standard.[69] Several green leaders, from the Dutch Ministry of the Environment to Starbucks's Green Team, advise people to reduce their environmental harm by using reusable coffee cups.[70]
In March 2019, Maryland banned polystyrene foam food containers and became the first state in the country to pass a food container foam ban through the state legislature. Maine was the first state to officially get a foam food container ban onto the books. In May 2019, Maryland Governor Hogan allowed the foam ban (House Bill 109) to become law without a signature making Maryland the second state to have a food container foam ban on the books, but is the first one to take effect on July 1, 2020.[71][72][73][74]
Outside the United States
China banned expanded polystyrene takeout/takeaway containers and tableware around 1999. However, compliance has been a problem and, in 2013, the Chinese plastics industry was lobbying for the ban's repeal.[75]
India and Taiwan also banned polystyrene-foam food-service ware before 2007.[76]
The government of Zimbabwe, through its Environmental Management Agency (EMA), banned polystyrene containers (popularly called 'kaylite' in the country), under Statutory Instrument 84 of 2012 (Plastic Packaging and Plastic Bottles) (Amendment) Regulations, 2012 (No 1.) [77] [78]
The city of Vancouver, Canada, has announced its Zero Waste 2040 plan in 2018. The city will introduce bylaw amendments to prohibit business license holders from serving prepared food in polystyrene foam cups and take-out containers, beginning 1 June 2019.[79]
Recycling

In general, polystyrene is not accepted in curbside collection recycling programs and is not separated and recycled where it is accepted. In Germany, polystyrene is collected, as a consequence of the packaging law (Verpackungsverordnung) that requires manufacturers to take responsibility for recycling or disposing of any packaging material they sell.
Most polystyrene products are currently not recycled due to the lack of incentive to invest in the compactors and logistical systems required. Due to the low density of polystyrene foam, it is not economical to collect. However, if the waste material goes through an initial compaction process, the material changes density from typically 30 kg/m3 to 330 kg/m3 and becomes a recyclable commodity of high value for producers of recycled plastic pellets. Expanded polystyrene scrap can be easily added to products such as EPS insulation sheets and other EPS materials for construction applications; many manufacturers cannot obtain sufficient scrap because of collection issues. When it is not used to make more EPS, foam scrap can be turned into products such as clothes hangers, park benches, flower pots, toys, rulers, stapler bodies, seedling containers, picture frames, and architectural molding from recycled PS.[80] As of 2016, around 100 tonnes of EPS are recycled every month in the UK.[81]
Recycled EPS is also used in many metal casting operations. Rastra is made from EPS that is combined with cement to be used as an insulating amendment in the making of concrete foundations and walls. American manufacturers have produced insulating concrete forms made with approximately 80% recycled EPS since 1993.
Incineration
If polystyrene is properly incinerated at high temperatures (up to 1000 °C[82]) and with plenty of air[82] (14 m3/kg), the chemicals generated are water, carbon dioxide, and possibly small amounts of residual halogen-compounds from flame-retardants.[82] If only incomplete incineration is done, there will also be leftover carbon soot and a complex mixture of volatile compounds.[83] According to the American Chemistry Council, when polystyrene is incinerated in modern facilities, the final volume is 1% of the starting volume; most of the polystyrene is converted into carbon dioxide, water vapor, and heat. Because of the amount of heat released, it is sometimes used as a power source for steam or electricity generation.[82][84]
When polystyrene was burned at temperatures of 800–900 °C (the typical range of a modern incinerator), the products of combustion consisted of "a complex mixture of polycyclic aromatic hydrocarbons (PAHs) from alkyl benzenes to benzoperylene. Over 90 different compounds were identified in combustion effluents from polystyrene."[85] The American National Bureau of Standards Center for Fire Research found 57 chemical by-products released during the combustion of expanded polystyrene (EPS) foam.[86]
Safety
Health
The American Chemistry Council, formerly known as the Chemical Manufacturers' Association, writes:
Based on scientific tests over five decades, government safety agencies have determined that polystyrene is safe for use in foodservice products. For example, polystyrene meets the stringent standards of the U.S. Food and Drug Administration and the European Commission/European Food Safety Authority for use in packaging to store and serve food. The Hong Kong Food and Environmental Hygiene Department recently reviewed the safety of serving various foods in polystyrene foodservice products and reached the same conclusion as the U.S. FDA.[87]
From 1999 to 2002, a comprehensive review of the potential health risks associated with exposure to styrene was conducted by a 12-member international expert panel selected by the Harvard Center for Risk Assessment. The scientists had expertise in toxicology, epidemiology, medicine, risk analysis, pharmacokinetics, and exposure assessment. The Harvard study reported that styrene is naturally present in trace quantities in foods such as strawberries, beef, and spices, and is naturally produced in the processing of foods such as wine and cheese. The study also reviewed all the published data on the quantity of styrene contributing to the diet due to migration of food packaging and disposable food contact articles, and concluded that risk to the general public from exposure to styrene from foods or food-contact applications (such as polystyrene packaging and foodservice containers) was at levels too low to produce adverse effects.[88]
Polystyrene is commonly used in containers for food and drinks. The styrene monomer (from which polystyrene is made) is a cancer suspect agent.[89] Styrene is "generally found in such low levels in consumer products that risks aren't substantial".[90] Polystyrene which is used for food contact may not contain more than 1% (0.5% for fatty foods) of styrene by weight.[91] Styrene oligomers in polystyrene containers used for food packaging have been found to migrate into the food.[92] Another Japanese study conducted on wild-type and AhR-null mice found that the styrene trimer, which the authors detected in cooked polystyrene container-packed instant foods, may increase thyroid hormone levels.[93]
Whether polystyrene can be microwaved with food is controversial. Some containers may be safely used in a microwave, but only if labeled as such.[94] Some sources suggest that foods containing carotene (vitamin A) or cooking oils must be avoided.[95]
Because of the pervasive use of polystyrene, these serious health related issues remain topical.[96]
Fire hazards
Like other organic compounds, polystyrene is flammable. Polystyrene is classified according to DIN4102 as a "B3" product, meaning highly inflammable or "Easily Ignited." As a consequence, although it is an efficient insulator at low temperatures, its use is prohibited in any exposed installations in building construction if the material is not flame-retardant. It must be concealed behind drywall, sheet metal, or concrete.[97] Foamed polystyrene plastic materials have been accidentally ignited and caused huge fires and losses of life, for example at the Düsseldorf International Airport and in the Channel Tunnel (where polystyrene was inside a railway carriage that caught fire).[98]
See also
- Styrofoam; brand of extruded polystyrene foam
- Foam food container
- Bioplastic
- Geofoam
- Structural insulated panel
- Polystyrene sulfonate
- Shrinky Dinks, a children's toy and activity kit consisting of sheets of polystyrene
References
- Wunsch, J.R. (2000). Polystyrene – Synthesis, Production and Applications. iSmithers Rapra Publishing. p. 15. ISBN 978-1-85957-191-0. Retrieved 25 July 2012.
- Wypych, George (2012). "PS polystyrene". Handbook of Polymers. pp. 541–7. doi:10.1016/B978-1-895198-47-8.50162-4. ISBN 978-1-895198-47-8.
- Haynes 2011, p. .
- Haynes 2011, pp. 13–17.
- John Scheirs; Duane Priddy (28 March 2003). Modern Styrenic Polymers: Polystyrenes and Styrenic Copolymers. John Wiley & Sons. p. 3. ISBN 978-0-471-49752-3.
- "Common Plastic Resins Used in Packaging". Introduction to Plastics Science Teaching Resources. American Chemistry Council, Inc. Retrieved 24 December 2012.
- Maul, J.; Frushour, B. G.; Kontoff, J. R.; Eichenauer, H.; Ott, K.-H. and Schade, C. (2007) "Polystyrene and Styrene Copolymers" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, doi:10.1002/14356007.a21_615.pub2
- Kwon BG, Saido K, Koizumi K, Sato H, Ogawa N, Chung SY, Kusui T, Kodera Y, Kogure K, et al. (May 2014). "Regional distribution of styrene analogues generated from polystyrene degradation along the coastlines of the North-East Pacific Ocean and Hawaii". Environmental Pollution. 188: 45–9. doi:10.1016/j.envpol.2014.01.019. PMID 24553245.
- Simon, E. (1839) "Ueber den flüssigen Storax (Styrax liquidus)" [On liquid storax (Styrax liquidus)], Annalen der Chemie, 31 : 265–277.
- , Blyth, John, and Hofmann, Aug. Wilh. (1845) "Ueber das Stryol und einige seiner Zersetzungsproducte" (On styrol and some of its decomposition products), Annalen der Chemie und Pharmacie, 53 (3) : 289–329.
- (Blyth and Hofmann, 1845), p. 312. From p. 312: (Analysis, as well as synthesis, have equally demonstrated, that styrol and the solid, glassy material, for which we suggest the name "meta styrol", possess the same percentage composition.)
- Berthelot, M. (1866) "Sur Les caractères de la benzine et du styrolène, comparés avec ceux des Autres carburetors d'hydrogène" (On the characters of benzene and styrene, compared with those of other hydrocarbons), Bulletin de la Société Chimique de Paris, 2nd series, 6: 289–298. From p. 294: "On sait que le stryolène chauffé en vase scellé à 200°, pendant Quelques heures, se change en un polymère résineux (métastyrol), et que ce polymère, distillé brusquement, reproduit le styrolène." (One knows that styrene [when] heated in a sealed vessel at 200°C, for several hours, is changed into a resinous polymer (polystyrene), and that this polymer, [when] distilled abruptly, reproduces styrene.)
- "Otis Ray McIntire". National Inventor's Hall of Fame.
- "Styrofoam, a Practical and Problematic Creation". Science History Institute. 31 July 2018.
- Natta, G.; Corradini, P.; Bassi, I. W. (1960). "Crystal structure of isotactic polystyrene". Il Nuovo Cimento. 15 (S1): 68–82. Bibcode:1960NCim...15S..68N. doi:10.1007/BF02731861.
- Ferrigno, T.H. (1967) Rigid Plastics Foams, 2nd edition. p. 207.
- "Celebrating 50 Years of Excellence in People and Products". Dart Container Corporation. Archived from the original on 4 June 2010. Retrieved 23 December 2012.
- "XAREC Syndiotactic Polystyrene – Petrochemicals – Idemitsu Kosan Global". www.idemitsu.com. Retrieved 1 January 2016.
- "What is Pyrolysis?". AZoCleantech.com. 29 December 2012. Retrieved 15 August 2019.
- Ho, Ba Thanh; Roberts, Timothy K.; Lucas, Steven (August 2017). "An overview on biodegradation of polystyrene and modified polystyrene: the microbial approach". Critical Reviews in Biotechnology. 38 (2): 308–320. doi:10.1080/07388551.2017.1355293. PMID 28764575.
- Jordan, R. (29 September 2015). "Plastic-eating worms may offer solution to mounting waste, Stanford researchers discover". Stanford News Service. Stanford University. Retrieved 4 January 2017.
- Yang Y, Yang J, Wu WM, Zhao J, Song Y, Gao L, Yang R, Jiang L (October 2015). "Biodegradation and Mineralization of Polystyrene by Plastic-Eating Mealworms: Part 1. Chemical and Physical Characterization and Isotopic Tests". Environmental Science & Technology. 49 (20): 12080–6. Bibcode:2015EnST...4912080Y. doi:10.1021/acs.est.5b02661. PMID 26390034.
- "Think you can't compost styrofoam? Mealworms are the answer!". Blog. Living Earth Systems. 8 October 2016. Retrieved 4 January 2017.
- Aumentado, Dominic. "A Comparative Study of the Efficacy of Tenebrio molitor Larvae and Zophobas morio Larvae as Degradation Agents of Expanded Polystyrene Foam". Academia.
- Roy, Robert (7 March 2006). "Immortal Polystyrene Foam Meets its Enemy". LiveScience. Retrieved 17 January 2019.
- Ward PG, Goff M, Donner M, Kaminsky W, O'Connor KE (April 2006). "A two step chemo-biotechnological conversion of polystyrene to a biodegradable thermoplastic". Environmental Science & Technology. 40 (7): 2433–7. Bibcode:2006EnST...40.2433W. doi:10.1021/es0517668. PMID 16649270.
- Biello, David (27 February 2006). "Bacteria Turn Styrofoam into Biodegradable Plastic". Scientific American.
- Goodier, K. (22 June 1961). "Making and using an expanded plastic". New Scientist. 240: 706.
- Mark, James E. (2009). Polymer Data Handbook (2nd Edition). Oxford University Press. ISBN 978-0-19-518101-2
- van der Vegt, A.K. and Govaert, L.E. (2003) Polymeren, van keten tot kunstof, DUP Blue Print, ISBN 90-407-2388-5
- Doroudiani, Saeed; Kortschot, Mark T. (2016). "Expanded Wood Fiber Polystyrene Composites: Processing–Structure–Mechanical Properties Relationships". Journal of Thermoplastic Composite Materials. 17: 13–30. doi:10.1177/0892705704035405.
- Doroudiani, Saeed; Chaffey, Charles E.; Kortschot, Mark T. (2002). "Sorption and diffusion of carbon dioxide in wood-fiber/polystyrene composites". Journal of Polymer Science Part B: Polymer Physics. 40 (8): 723–735. Bibcode:2002JPoSB..40..723D. doi:10.1002/polb.10129.
- Mihai, Mihaela; Huneault, Michel A.; Favis, Basil D. (2016). "Foaming of Polystyrene/ Thermoplastic Starch Blends". Journal of Cellular Plastics. 43 (3): 215–236. doi:10.1177/0021955X07076532.
- Norton, Jed. "Blue Foam, Pink Foam and Foam Board". Antenociti's Workshop. Archived from the original on 26 February 2008. Retrieved 29 January 2008.
- "Polystyrene". ChemicalSafetyFacts.org. American Chemistry Council. May 2014.
- "Recycle Your EPS". EPS Industry Alliance. Retrieved 11 December 2017.
- "Products: graphite enhanced polystyrene". Neotherm Ltd. Archived from the original on 11 March 2018. Retrieved 26 December 2018.
- Expanded Polystyrene (EPS) Technical Data (PDF). Australia: Australian Urethane & Styrene. 2010.
- "Moulding Expanded Polystyrene (EPS)".
- "Dow Chemical Company Styrofoam page". Archived from the original on 24 March 2008. Retrieved 17 January 2019.
- Expandable Polystyrene, Insight database from Ceresana Research
- "Technical details". Depron foam. Retrieved 17 June 2020.
- Gnip, Ivan et al. (2007) LONG-TERM WATER ABSORPTION OF EXPANDED POLYSTYRENE BOARDS Archived 28 January 2018 at the Wayback Machine. Institute of Thermal Insulation of Vilnius Gediminas Technical University
- Owens Corning FOAMULAR Extruded Polystrene Insulation: Resisting Water Absorption, the Key for High-Performance Foam Plastic Rigid Insulation, Technical Bulletin, Pub. No. 10011642-A, September 2011,
- "XPS Insulation Extracted After Field Exposure Confirms High Water Absorption & Diminished R‐Value", EPS Below Grade Series 105, March 2014, Technical Bulletin, EPS Industry Alliance.
- W. Keim: Kunststoffe: Synthese, Herstellungsverfahren, Apparaturen, 379 Seiten, Verlag Wiley-VCH Verlag GmbH & Co. KGaA, 1. Auflage (2006) ISBN 3-527-31582-9
- "Übersicht Polystyrol auf chemgapedia.de".
- Domininghaus, Hans. (2012). Kunststoffe : Eigenschaften und Anwendungen. Elsner, Peter., Eyerer, Peter., Hirth, Thomas. (8., neu bearbeitete und erweiterte Auflage ed.). Heidelberg: Springer. ISBN 9783642161735. OCLC 834590709.
- "Schlagzähes PS auf chemgapedia.de".
- Jürgen Maul, Bruce G. Frushour, Jeffrey R. Kontoff, Herbert Eichenauer, Karl-Heinz Ott, Christian Schade (2007). "Polystyrene and Styrene Copolymers". Ullmanns Enzyklopädie der Technischen Chemie. doi:10.1002/14356007.a21_615.pub2. ISBN 978-3527306732.CS1 maint: multiple names: authors list (link)
- "PS-Pfropfcopolymere auf chemgapedia.de".
- "PS-Blockcopolymere auf chemgapedia.de".
- "styrenic block copolymers - IISRP" (PDF).
- Polystyrene Foam Report Archived 25 March 2013 at the Wayback Machine. Earth Resource Foundation.
- Global Warming Potentials of ODS Substitutes. EPA.gov
- Bandyopadhyay, A.; Basak, G. Chandra (2013). "Studies on photocatalytic degradation of polystyrene". Materials Science and Technology. 23 (3): 307–314. doi:10.1179/174328407X158640.
- Hofer, Tobias N. (2008). Marine pollution : new research. New York: Nova Science Publishers. p. 59. ISBN 978-1-60456-242-2.
- Schnurr, Riley E.J.; Alboiu, Vanessa; Chaudhary, Meenakshi; Corbett, Roan A.; Quanz, Meaghan E.; Sankar, Karthikeshwar; Srain, Harveer S.; Thavarajah, Venukasan; Xanthos, Dirk; Walker, Tony R. (2018). "Reducing marine pollution from single-use plastics (SUPs): A review". Marine Pollution Bulletin. 137: 157–171. doi:10.1016/j.marpolbul.2018.10.001. PMID 30503422.
- "Berkeley Barring Use Of a Food Container". The New York Times. Associated Press. 24 September 1987. Retrieved 23 December 2012.
- "Suffolk Votes A Bill to Ban Plastic Bags". New York Times. 30 March 1988. Retrieved 23 December 2012.
- Hevesi, Dennis (4 March 1990). "Ban on Plastics in Suffolk Is Overturned". The New York Times. Retrieved 23 December 2012.
- Barbanel, Josh (4 March 1992). "Vote Blocks Plastics Ban For Suffolk". The New York Times. Retrieved 23 December 2012.
- "Berkeley Widens Ban on Foam Food Containers". The Los Angeles Times. 16 June 1988. Retrieved 23 December 2012.
- Herron Zamora, Jim (28 June 2006). "Styrofoam food packaging banned in Oakland". San Francisco Chronicle. Retrieved 23 December 2012.
- Sanchez, Kris (27 August 2013). "San Jose Approves Styrofoam Ban". NBC. Retrieved 30 August 2013.
- "CHAPTER 33 STYROFOAM ORDINANCE". Ordinances. Town of Freeport, Maine. Retrieved 23 December 2012.
- Tony Dokoupil (22 September 2015). "msnbc.com". msnbc.com. Retrieved 17 January 2019.
- "S.F. supervisors OK toughest ban on foam packaging in U.S". 30 June 2016. Retrieved 30 June 2016.
- "Disposables Standard". Green Restaurant Association. Retrieved 14 December 2016.
- Dineen, Shauna (November–December 2005). "The Throwaway Generation: 25 Billion Styrofoam Cups a Year". E-The Environmental Magazine. Archived from the original on 12 November 2006.
- Andrew M. Ballard. "Maryland Foam Packaging Ban, Energy Bills to Become Law". news.bloombergenvironment.com. Retrieved 20 June 2019.
- "Statement: Maryland becomes the second state to ban plastic foam containers". environmentamerica.org. Retrieved 20 June 2019.
- Sun, Baltimore. "Maryland's new laws: banning foam food containers, raising tobacco-buying age, reforming UMMS board". baltimoresun.com. Retrieved 20 June 2019.
- "2019 Foam Ban". Maryland League of Conservation Voters. 30 May 2019. Retrieved 20 June 2019.
- Ying Sun, Nina & Toloken, Steve (21 March 2013). "China moves to end its 'ban' on PS food packaging". Plastics News. Plastics News. Retrieved 10 June 2013.
- Quan, Jean (13 June 2006). "letter to Public Works Committee" (PDF). Archived from the original (PDF) on 23 October 2006. Retrieved 26 January 2014.
- "Government bans kaylite packaging". The Herald. 13 July 2017. Retrieved 13 July 2017.
- "Expanded polystyrene (kaylite): What are its impacts?". The Herald. 12 July 2017. Retrieved 13 July 2017.
- Single-Use Item Reduction Strategy, Zero Waste 2040 City of Vancouver, 2018
- https://expandedpoly.co.uk/environment/ Polystyrene recycling. Retrieved 17 October 2019.
- EPS recycling. Eccleston & Hart Polystrene. Retrieved 21 July 2016.
- BASF Technische Information TI 0/2-810d 81677 Juni 1989, Verwertungs- und Beseitigungsverfaren gebrauchter Schaumstoff-Verpackungen aus Styropor®
- Polystyrene Foam Burning Danger. Newton.dep.anl.gov. Retrieved 25 December 2011. Q and A page with an partially incorrect information.
- "Ease of Disposal". Archived from the original on 7 June 2009. Retrieved 25 June 2009.
- Hawley-Fedder, R.A.; Parsons, M.L.; Karasek, F.W. (1984). "Products obtained during combustion of polymers under simulated incinerator conditions". Journal of Chromatography A. 315: 201–210. doi:10.1016/S0021-9673(01)90737-X. Quoted from a campaign site giving no details of the original source and experiment conditions.
- "highcountryconservation.org" (PDF). Archived from the original (PDF) on 15 September 2012. Retrieved 9 August 2018.
- "Q & A on the Safety of Polystyrene Foodservice Products". American Chemistry Council. 2010–2011. Archived from the original on 24 August 2011. Retrieved 14 June 2011.
- Cohen JT; Carlson G; Charnley G; Coggon D; Delzell E; Graham JD; Greim H; Krewski D; Medinsky M; Monson R; Paustenbach D; Petersen B; Rappaport S; Rhomberg L; Ryan PB; Thompson K (2011). "A comprehensive evaluation of the potential health risks associated with occupational and environmental exposure to styrene". Journal of Toxicology and Environmental Health Part B: Critical Reviews. 5 (1–2): 1–265. doi:10.1080/10937400252972162. Lay summary – The McLaughlin Centre for Population Health Risk Assessment.
- National Toxicology Program (10 June 2011). "12th Report on Carcinogens". National Toxicology Program. Archived from the original on 12 June 2011. Retrieved 11 June 2011.
- Harris, Gardiner (10 June 2011). "Government Says 2 Common Materials Pose Risk of Cancer". New York Times. Retrieved 11 June 2011.
- "Sec. 177.1640 Polystyrene and rubber-modified polystyrene". Code of Federal Regulations, Title 21—Food and Drugs, Subchapter B—Food for Human Consumption. U.S. Food and Drug Administration. Retrieved 4 April 2014.
- Sakamoto, Hiromi; Matsuzaka, Ayako; Itoh, Rimiko; Tohyama, Yuko (2000). "使い捨て弁当容器から溶出するスチレンダイマー及びトリマーの定量" [Quantitative Analysis of Styrene Dimer and Trimers Migrated from Disposable Lunch Boxes]. Journal of the Food Hygienic Society of Japan (in Japanese). 41 (3): 200–205. doi:10.3358/shokueishi.41.200.
- Yanagiba Y, Ito Y, Yamanoshita O, Zhang SY, Watanabe G, Taya K, Li CM, Inotsume Y, Kamijima M, Gonzalez FJ, Nakajima T (June 2008). "Styrene trimer may increase thyroid hormone levels via down-regulation of the aryl hydrocarbon receptor (AhR) target gene UDP-glucuronosyltransferase". Environmental Health Perspectives. 116 (6): 740–5. doi:10.1289/ehp.10724. PMC 2430229. PMID 18560529.
- "Microwaving food in plastic: Dangerous or not?". Harvard Health. 20 September 2017.
- "Polystyrene & Health Homepage". Energy Justice Network. Retrieved 9 December 2013.
- Entine, Jon (14 September 2011). "Styrene in the Crosshairs: Competing Standards Confuse Public, Regulators". American Enterprise Institute.
- Nelligan, R.J. (2006). Guidelines for the use of expanded foam polystyrene panel systems in industrial buildings to minimize the risk of fire (PDF) (MS Thesis). OCLC 166313665.
- "Foul Play Considered in Channel Tunnel Fire Inquiry". The Irish Times. 28 November 1996. Retrieved 14 January 2018.
Bibliography
- Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). CRC Press. ISBN 978-1439855119.CS1 maint: ref=harv (link)
External links
| Wikimedia Commons has media related to Polystyrene. |
- Polystyrene Composition – The University of Southern Mississippi
- SPI resin identification code – Society of the Plastics Industry
- Polystyrene: Local Ordinances – Californians Against Waste
- Take a Closer Look at Today’s Polystyrene Packaging (brochure by the industry group American Chemistry Council, arguing that the material is "safe, affordable and environmentally responsible")
- Lettieri TR, Hartman AW, Hembree GG, Marx E (1991). "Certification of SRM1960: Nominal 10 μm Diameter Polystyrene Spheres ("Space Beads")". Journal of Research of the National Institute of Standards and Technology. 96 (6): 669–691. doi:10.6028/jres.096.044. PMC 4915770. PMID 28184141.
- Polystyrene Biodegradation – BioSphere Plastic