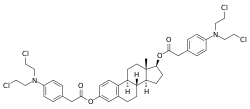
Estradiol mustard
Estradiol mustard, also known as chlorphenacyl estradiol diester, as well as estradiol 3,17β-bis(4-(bis(2-chloroethyl)amino)phenyl)acetate, is a synthetic, steroidal estrogen and cytostatic antineoplastic agent and a chlorphenacyl nitrogen mustard-coupled estrogen ester that was never marketed.[1] It is selectively distributed into estrogen receptor (ER)-positive tissues such as ER-expressing tumors like those seen in breast and prostate cancers.[2] For this reason, estradiol mustard and other cytostatic-linked estrogens like estramustine phosphate have reduced toxicity relative to non-linked nitrogen mustard cytostatic antineoplastic agents.[2] However, they may stimulate breast tumor growth due to their inherent estrogenic activity and are said to be devoid of major therapeutic efficacy in breast cancer,[3] although estramustine phosphate has been approved for and is used (almost exclusively) in the treatment of prostate cancer.[4]
 | |
| Clinical data | |
|---|---|
| Other names | NSC-112259; Chlorphenacyl estradiol diester; Estradiol 3,17β-bis(4-(bis(2-chloroethyl)amino)phenyl)acetate |
| Drug class | Chemotherapeutic agent; Estrogen; Estrogen ester |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C42H50Cl4N2O4 |
| Molar mass | 788.67 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
See also
References
- J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 898–. ISBN 978-1-4757-2085-3.
- Asai M, Takeuchi H, Okada H (1978). "In vivo interaction between steroidal alkylating agents and oestrogen receptors in rabbit uteri". Acta Endocrinol. 87 (1): 173–80. doi:10.1530/acta.0.0870173. PMID 579532.
- V. H. T. James; J. R. Pasqualini (22 October 2013). Hormonal Steroids: Proceedings of the Sixth International Congress on Hormonal Steroids. Elsevier Science. pp. 75–. ISBN 978-1-4831-9067-9.
- Richard J. Ablin; Malcolm D. Mason (5 September 2007). Metastasis of Prostate Cancer. Springer Science & Business Media. pp. 311–. ISBN 978-1-4020-5847-9.