Tacalcitol
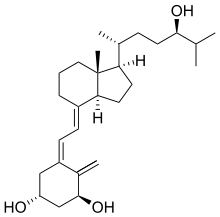
Tacalcitol (1,24-dihydroxyvitamin D3) is a synthetic vitamin D3 analog.[1]
 | |
| Clinical data | |
|---|---|
| Other names | (1α,24R)-1,24-Dihydroxyvitamin D3 |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Topical |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.220.855 |
| Chemical and physical data | |
| Formula | C27H44O3 |
| Molar mass | 416.64 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Tacalcitol is marketed under several names, including Curatoderm and Bonalfa.
Mechanism
Tacalcitol reduces excessive cell turnover in the epidermis by interacting with vitamin D receptors on keratinocytes.
Uses
It is usually prescribed by a general practitioner or dermatologist for the treatment of psoriasis, chronic chapped lips and other severe dry skin conditions because of its ability to reduce excessive skin cell turnover. It is available as an ointment or lotion.
It has also been used for vitiligo[2] and Hailey-Hailey disease.[3]
gollark: They aren't very good weapons for anything but warcrime maximization.
gollark: The police need *orbital bombardment*, obviously.
gollark: In what way?
gollark: Technically, with some ridiculous and implausible amount of resources and control of some of the internet and some broadcasters, you could totally fake this, for a while.
gollark: Both parties can gerrymander etc. horribly so I don't see much of an incentive for them to do that.
References
- Fukuoka M, Sakurai K, Ohta T, Kiyoki M, Katayama I (2001). "Tacalcitol, an active vitamin D3, induces nerve growth factor production in human epidermal keratinocytes". Skin Pharmacol. Appl. Skin Physiol. 14 (4): 226–33. doi:10.1159/000056351. PMID 11464105.
- Leone G, Pacifico A, Iacovelli P, Paro Vidolin A, Picardo M (March 2006). "Tacalcitol and narrow-band phototherapy in patients with vitiligo". Clin. Exp. Dermatol. 31 (2): 200–5. doi:10.1111/j.1365-2230.2005.02037.x. PMID 16487090.
- Aoki T, Hashimoto H, Koseki S, Hozumi Y, Kondo S (November 1998). "1alpha,24-dihydroxyvitamin D3 (tacalcitol) is effective against Hailey-Hailey disease both in vivo and in vitro". Br. J. Dermatol. 139 (5): 897–901. doi:10.1046/j.1365-2133.1998.02522.x. PMID 9892963.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.