Phytomenadione
Phytomenadione, also known as vitamin K1 or phylloquinone, is a vitamin found in food and used as a dietary supplement.[1][2] As a supplement it is used to treat certain bleeding disorders.[2] This includes warfarin overdose, vitamin K deficiency, and obstructive jaundice.[2] It is also recommended to prevent and treat vitamin K deficiency bleeding in infants.[2] Use is typically recommended by mouth or injection under the skin.[2] Use by injection into a vein or muscle is recommended only when other routes are not possible.[2] When given by injection benefits are seen within two hours.[2]
 | |
| Clinical data | |
|---|---|
| Trade names | Mephyton, others |
| Other names | Vitamin K1, phytonadione, phylloquinone, (E)-phytonadione |
| AHFS/Drugs.com | Monograph |
| Pregnancy category |
|
| Routes of administration | by mouth, subQ, IM, IV |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.001.422 |
| Chemical and physical data | |
| Formula | C31H46O2 |
| Molar mass | 450.707 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Common side effects when given by injection include pain at the site of injection and altered taste.[2] Severe allergic reactions may occur when it is injected into a vein or muscle.[2] It is unclear if use during pregnancy is safe; however, use is likely okay during breastfeeding.[3] It works by supplying a required component for making a number of blood clotting factors.[2] Found sources include green vegetables, vegetable oil, and some fruit.[4]
Phytomenadione was first isolated in 1939.[5] In 1943 Edward Doisy and Henrik Dam were given a Nobel Prize for its discovery.[5] It is on the World Health Organization's List of Essential Medicines.[6]
Terminology
Phytomenadione is often also called phylloquinone, vitamin K,[7] or phytonadione. Sometimes a distinction is made between phylloquinone, which is considered to be a natural substance, and phytonadione, which is considered to be a synthetic substance.[8]
A stereoisomer of phylloquinone is called vitamin k1 (note the difference in capitalization).
Chemistry
Vitamin K is a fat-soluble vitamin that is stable in air and moisture but decomposes in sunlight. It is a polycyclic aromatic ketone, based on 2-methyl-1,4-naphthoquinone, with a 3-phytyl substituent. It is found naturally in a wide variety of green plants, particularly in leaves, since it functions as an electron acceptor during photosynthesis, forming part of the electron transport chain of photosystem I.[9][10]
The best-known function of vitamin K in animals is as a cofactor in the formation of coagulation factors II (prothrombin), VII, IX, and X by the liver. It is also required for the formation of anticoagulant factors protein C and S. It is commonly used to treat warfarin toxicity, and as an antidote for coumatetralyl.
Vitamin K is required for bone protein formation.
Biosynthesis
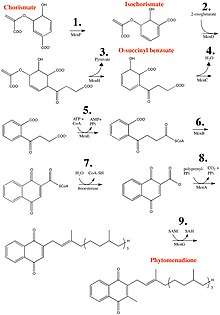
Vitamin K1 is synthesized from chorismate, a compound produced from shikimate via the shikimate pathway. The conversion of chorismate to vitamin K1 comprises a series of nine steps:[11][12]
- Chorismate is isomerized to isochorismate by isochorismate synthase, or MenF (menaquinone enzyme).
- Addition of 2-oxoglutarate to isochorismate by PHYLLO, a multifunctional protein comprising three different enzymatic activities (MenD, H, and C).
- Elimination of pyruvate by PHYLLO.
- Aromatization to yield o-succinyl benzoate by PHYLLO.
- O-succinylbenzoate activation to corresponding CoA ester by MenE.
- Naphthoate ring formation by naphthoate synthase (MenB/NS).
- Thiolytic release of CoA by a thioesterase (MenH).
- Attachment of phytol chain to the naphthoate ring (MenA/ABC4).
- Methylation of the precursor at position 3 (MenG).
See also
References
- Watson RR (2014). Diet and Exercise in Cystic Fibrosis. Academic Press. p. 187. ISBN 9780128005880. Archived from the original on 2016-12-30.
- "Phytonadione". The American Society of Health-System Pharmacists. Archived from the original on 29 December 2016. Retrieved 8 December 2016.
- "Phytonadione Use During Pregnancy". Drugs.com. Archived from the original on 29 December 2016. Retrieved 29 December 2016.
- "Office of Dietary Supplements – Vitamin K". ods.od.nih.gov. 11 February 2016. Archived from the original on 31 December 2016. Retrieved 30 December 2016.
- Sneader W (2005). Drug Discovery: A History. John Wiley & Sons. p. 243. ISBN 9780471899792. Archived from the original on 2016-12-30.
- World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- Haroon Y, Shearer MJ, Rahim S, Gunn WG, McEnery G, Barkhan P (June 1982). "The content of phylloquinone (vitamin K1) in human milk, cows' milk and infant formula foods determined by high-performance liquid chromatography". The Journal of Nutrition. 112 (6): 1105–17. doi:10.1093/jn/112.6.1105. PMID 7086539.
- "Vitamin K". Retrieved 2009-03-18.
- Itoh S, Iwaki M (1989). "Vitamin K1 (Phylloquinone) Restores the Turnover of FeS centers of Ether-extracted Spinach PSI Particles". FEBS Letters. 243 (1): 47–52. doi:10.1016/0014-5793(89)81215-3.
- Palace GP, Franke JE, Warden JT (May 1987). "Is phylloquinone an obligate electron carrier in photosystem I?". FEBS Letters. 215 (1): 58–62. doi:10.1016/0014-5793(87)80113-8. PMID 3552735.
- Reumann S (2013). "Biosynthesis of vitamin K1 (phylloquinone) by plant peroxisomes and its integration into signaling molecule synthesis pathways". Sub-Cellular Biochemistry. Subcellular Biochemistry. Springer Netherlands. 69: 213–29. doi:10.1007/978-94-007-6889-5_12. ISBN 9789400768888. PMID 23821151.
- Widhalm JR, van Oostende C, Furt F, Basset GJ (April 2009). "A dedicated thioesterase of the Hotdog-fold family is required for the biosynthesis of the naphthoquinone ring of vitamin K1". Proceedings of the National Academy of Sciences of the United States of America. 106 (14): 5599–603. doi:10.1073/pnas.0900738106. PMC 2660889. PMID 19321747.
External links
- "Phytomenadione". Drug Information Portal. U.S. National Library of Medicine.