Desmosterol
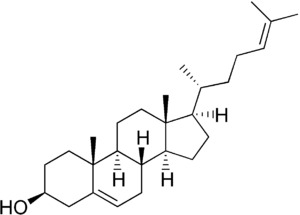
Desmosterol is a molecule similar to cholesterol. Desmosterol is the immediate precursor of cholesterol in the Bloch pathway of cholesterol biosynthesis.[1] 24-dehydrocholesterol reductase catalyses the reduction of desmosterol to cholesterol.[2] It is accumulated in desmosterolosis.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
(3S,8S,9S,10R,13R,14S,17R)-10,13-dimethyl-17-[(2R)-6-methylhept-5-en-2-yl]-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-ol | |
| Other names
Cholesta-5,24-dien-3β-ol | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.005.671 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C27H44O | |
| Molar mass | 384.64 g/mol |
| Appearance | White powder |
| Melting point | 121.5 °C (250.7 °F; 394.6 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
In 2014, it was named the Molecule of the Year 2012 by the International Society for Molecular and Cell Biology and Biotechnology Protocols and Researches (ISMCBBPR).[3]
See also
References
- Vainio, S.; Jansen, M.; Koivusalo, M.; Rog, T.; Karttunen, M.; Vattulainen, I.; Ikonen, E. (25 October 2005). "Significance of Sterol Structural Specificity: DESMOSTEROL CANNOT REPLACE CHOLESTEROL IN LIPID RAFTS". Journal of Biological Chemistry. 281 (1): 348–355. doi:10.1074/jbc.M509530200. PMID 16249181. Retrieved 30 June 2015.
- Keber, R.; Rozman, D.; Horvat, S. (23 October 2012). "Sterols in spermatogenesis and sperm maturation". The Journal of Lipid Research. 54 (1): 20–33. doi:10.1194/jlr.R032326. PMC 3520525. PMID 23093550. Retrieved 30 June 2015.
- Announcing ISMCBBPR's Molecule of the Year 2012
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.