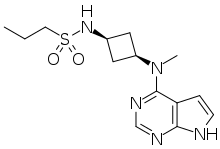
Abrocitinib
Abrocitinib (code name PF-04965842) is a Janus kinase inhibitor drug which is currently under investigation for the treatment of atopic dermatitis. It was developed by Pfizer.
 | |
| Clinical data | |
|---|---|
| Other names | PF-04965842 |
| Routes of administration | By mouth |
| Pharmacokinetic data | |
| Elimination half-life | 2.8–5.2 h |
| Excretion | 1.0–4.4% unchanged in urine |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| ECHA InfoCard | 100.251.498 |
| Chemical and physical data | |
| Formula | C14H21N5O2S |
| Molar mass | 323.42 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Side effects
The most common adverse effects in studies were upper respiratory tract infection, headache, nausea, and diarrhea.[1]
Pharmacology
Mechanism of action
It is a selective inhibitor of the enzyme janus kinase 1 (JAK1).[1]
Pharmacokinetics
Abrocitinib is quickly absorbed from the gut and generally reaches highest blood plasma concentrations within one hour. Only 1.0 to 4.4% of the dose are found unmetabolized in the urine.[2]
Timeline
- April 2016: initiation of Phase 2b trial
- December 2017: initiation of JADE Mono-1 Phase 3 trial[3]
- May 2018: Results of Phase 2b trial posted
- October 2019: Results of Phase 3 trial presented[4]
- June 2020: Results of second Phase 3 trial published[5]
gollark: ↑
gollark: It was created when when.
gollark: It's not an actual database itself.
gollark: SQL is *for* relational databases.
gollark: Or postgreSQL.
References
- Gooderham MJ, Forman SB, Bissonnette R, Beebe JS, Zhang W, Banfield C, et al. (October 2019). "Efficacy and Safety of Oral Janus Kinase 1 Inhibitor Abrocitinib for Patients With Atopic Dermatitis: A Phase 2 Randomized Clinical Trial". JAMA Dermatology. doi:10.1001/jamadermatol.2019.2855. PMC 6777226. PMID 31577341.
- Peeva E, Hodge MR, Kieras E, Vazquez ML, Goteti K, Tarabar SG, et al. (August 2018). "Evaluation of a Janus kinase 1 inhibitor, PF-04965842, in healthy subjects: A phase 1, randomized, placebo-controlled, dose-escalation study". British Journal of Clinical Pharmacology. 84 (8): 1776–1788. doi:10.1111/bcp.13612. PMC 6046510. PMID 29672897.
- Clinical trial number NCT03349060 for "Study to Evaluate Efficacy and Safety of PF-04965842 in Subjects Aged 12 Years And Older With Moderate to Severe Atopic Dermatitis (JADE Mono-1)" at ClinicalTrials.gov
- "Pfizer Presents Positive Phase 3 Data at the 28th Congress of the European Academy of Dermatology and Venereology for Abrocitinib in Moderate to Severe Atopic Dermatitis". Drugs.com. 2019-10-12.
- "Efficacy and Safety of Abrocitinib in Patients With Moderate-to-Severe Atopic Dermatitis: A Randomized Clinical Trial". JAMA Dermatology. June 3, 2020. doi:10.1001/jamadermatol.2020.1406. Retrieved June 4, 2020. Cite journal requires
|journal=(help)
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.