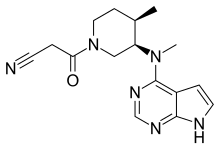
Tofacitinib
Tofacitinib, sold under the brand Xeljanz among others, is a medication used to treat rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and ulcerative colitis.[2][3]
 | |
| Clinical data | |
|---|---|
| Trade names | Xeljanz, Jakvinus, Tofacinix, Others |
| Other names | CP-690550 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a613025 |
| License data |
|
| Pregnancy category | |
| Routes of administration | By mouth (tablets) |
| Drug class | Janus kinase (JAK) inhibitor |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 74% |
| Protein binding | 40% |
| Metabolism | Liver (via CYP3A4 and CYP2C19) |
| Elimination half-life | 3 hours |
| Excretion | Urine |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| PubChem SID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.215.928 |
| Chemical and physical data | |
| Formula | C16H20N6O |
| Molar mass | 312.377 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Common side effects include diarrhea, headache, and high blood pressure.[2] Serious side effects may include infections, cancer, and pulmonary embolism.[2][4] In 2019, the safety committee of the European Medicines Agency (EMA) began a review of tofacitinib and recommended that doctors temporarily not prescribe the 10 mg twice-daily dose to people at high risk for pulmonary embolism.[5] The U.S. Food and Drug Administration (FDA) also released warnings about the risk of blood clots.[6][7][8]
It is in the janus kinase (JAK) inhibitor class, discovered and developed by the National Institutes of Health and Pfizer.
Medical uses
Rheumatoid arthritis
In November 2012, the U.S. Food and Drug Administration (FDA) approved tofacitinib citrate "to treat adults with moderately to severely active rheumatoid arthritis who have had an inadequate response to, or who are intolerant of, methotrexate."[9] It was later approved in Japan, Switzerland and others (but not the EU). It is marketed as Xeljanz in all regions except for Russia where it will be marketed as Jakvinus or Jaquinus.[10]
Ulcerative colitis
In May 2018, the U.S. FDA approved tofacitinib citrate "for the treatment of adult patients in the U.S. with moderately to severely active ulcerative colitis."[3] Tofacitinib citrate is the first oral JAK inhibitor approved for chronic use in ulcerative colitis (tofacitinib is a small molecule, not a biologic).
Adverse effects
Tofacitinib was initially not approved by European regulatory agencies because of concerns over efficacy and safety,[11] although by 2018 the European Commission had approved it.[12] Animal studies with tofacitinib conducted prior to human trials showed some carcinogenesis, mutagenesis, and impairment of fertility.[13]
The most commonly reported adverse reactions during the first three months in controlled clinical trials (occurring in greater than or equal to 2% of patients treated with tofacitinib citrate monotherapy or in combination with DMARDs) were upper respiratory tract infections, headache, diarrhea, and nasopharyngitis (the "common cold").[13]
Tofacitinib is required by the U.S. Food and Drug Administration (FDA) to have a boxed warning on its label about possible injury and death due to problems such as infections, lymphoma and other malignancies which can arise from use of this drug.[9] Serious infections leading to hospitalization or death, including tuberculosis and bacterial, invasive fungal, viral, and other opportunistic infections, have occurred in patients receiving tofacitinib. Epstein Barr Virus-associated post-transplant lymphoproliferative disorder has been observed at an increased rate in renal transplant patients treated with tofacitinib while on immunosuppressive medications. Patients are warned to avoid use of tofacitinib citrate during an "active serious infection, including localized infections." Doctors are advised to use it with caution in patients that may be at increased risk of gastrointestinal perforations. Laboratory monitoring is recommended due to potential changes in lymphocytes, neutrophils, hemoglobin, liver enzymes and lipids. Tofacitinib claims to have no contraindications, however doctors are advised to reduce the patient's dosage when combined with "potent inhibitors of Cytochrome P450 3A4 (CYP3A4)," such as ketoconazole, or one or more combined medications that result in both moderate inhibition of CYP3A4 and potent inhibition of CYP2C19 such as fluconazole. Furthermore, immunizations with live vaccines should be avoided by tofacitinib users.[13]
According to post-marketing research, tofacitinib may also increase the risk for pulmonary embolism. Prescribers should consider risk factors for pulmonary embolism before prescribing this medication. Risk factors include age, obesity, smoking and immobilization. Patients taking this medication, irrespective of indication or risk factors, should be monitored for signs and symptoms of pulmonary embolism.[14]
Mechanism
It is an inhibitor of the enzyme janus kinase 1 (JAK1) and janus kinase 3 (JAK 3), which means that it interferes with the JAK-STAT signaling pathway, which transmits extracellular information into the cell nucleus, influencing DNA transcription.[15]
In a mouse model of established arthritis, tofacitinib rapidly improved disease by inhibiting the production of inflammatory mediators and suppressing STAT1-dependent genes in joint tissue. This efficacy in this disease model correlated with the inhibition of both JAK1 and 3 signaling pathways, suggesting that tofacitinib may exert therapeutic benefit via pathways that are not exclusive to inhibition of JAK3.[16]
History
The potential significance of JAK3 inhibition was first discovered in the laboratory of John O'Shea, an immunologist at the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (NIH).[17] In 1994, Pfizer was approached by the NIH to form a public-private partnership in order to evaluate and bring to market experimental compounds based on this research.[17] Pfizer initially declined the partnership but agreed in 1996, after the elimination of an NIH policy dictating that the market price of a product resulting from such a partnership would need to be commensurate with the investment of public taxpayer revenue and the "health and safety needs of the public."[17] Pfizer worked with O'Shea's laboratory to define the structure and function of JAK3 and its receptors, and then handled the drug discovery, preclinical development, and clinical development of tofacitinib in-house.[18]
The drug was coded as CP-690,550[19] during development. Its original recommended INN (rINN) was tasocitinib,[20] but that was overruled during the INN approval process as being inoptimally differentiable from other existing INNs, so the name tofacitinib was proposed and became the INN.
In November 2012, the U.S. Food and Drug Administration (FDA) approved tofacitinib for treatment of rheumatoid arthritis. Two rheumatologists interviewed by the magazine Nature Biotechnology complained that they were "shocked" and "disappointed" at the $2,055 a month wholesale price.[18]
A 2014 study showed that tofacitinib treatment was able to convert white fat tissues into more metabolically active brown fat, suggesting it may have potential applications in the treatment of obesity.[21]
In November 2012, the FDA approved tofacitinib "to treat adults with moderately to severely active rheumatoid arthritis who have had an inadequate response to, or who are intolerant of, methotrexate.[9] The FDA approved only the 5 mg twice-daily dose on the grounds that a higher dose was not considered to have an adequate risk-to-benefit ratio.[22]
Research
It has demonstrated effectiveness in the treatment of psoriasis in Phase 3 studies. It is being studied for treatment of inflammatory bowel disease,[23][24] and other immunological diseases, as well as for the prevention of organ transplant rejection.[25][26][27][28]
Psoriasis
Tofacitinib is a current investigational drug in psoriasis. Tofacitinib has demonstrated its effectiveness for plaque psoriasis in Phase 3 randomized, controlled trials in comparison to placebo and to etanercept.[22][29][30] In particular, a 10 mg twice-daily dose of tofacitinib was shown to be noninferior to etanercept 50 mg subcutaneously twice weekly.[30] Approval of tofacitinib for the treatment of psoriasis was rejected by the FDA due to safety concerns.[31]
Alopecia areata
Based on preclinical studies in a mouse model of the disease,[32] tofacitinib has been investigated for the treatment of alopecia areata. Early case reports[33][34] suggested potential efficacy, as did a phase II open-label clinical trial,[35] published in tandem with a phase II clinical trial showing the same for ruxolitinib.[36]
Vitiligo
In a June 2015 case report, a 53-year-old woman with vitiligo showed noticeable improvement after taking tofacitinib for five months.[37]
Atopic dermatitis
The results of using tofacitinib in six patients with recalcitrant atopic dermatitis was published in the September 2015. All saw improvement in their atopic dermatitis without any adverse events.[38]
Ankylosing spondylitis
As of 2016 it is undergoing a phase II trial for ankylosing spondylitis.[39]
References
- Use During Pregnancy and Breastfeeding
- "Tofacitinib Citrate". The American Society of Health-System Pharmacists. Retrieved 1 June 2018.
- "FDA approves new treatment for moderately to severely active ulcerative colitis". U.S. Food and Drug Administration (FDA) (Press release). 30 May 2018. Archived from the original on 15 December 2019. Retrieved 1 June 2018.

- "Safety Alerts for Human Medical Products - Xeljanz, Xeljanz XR (tofacitinib): Safety Communication - Safety Trial Finds Increased Risk of Blood Clots in the Lungs and Death with Higher Dose in Rheumatoid Arthritis Patients". U.S. Food and Drug Administration (FDA). Retrieved 2 March 2019.

- "Meeting highlights from the Pharmacovigilance Risk Assessment Committee (PRAC) 13-16 May 2019, May 17, 2019". European Medicines Agency (EMA). Retrieved 17 May 2019.
- "Xeljanz, Xeljanz XR (tofacitinib): Drug Safety Communication - Due to an Increased Risk of Blood Clots and Death with Higher Dose". U.S. Food and Drug Administration (FDA). 26 July 2019. Archived from the original on 15 December 2019. Retrieved 10 August 2019.

- FDA approves Boxed Warning about increased risk of blood clots and death with higher dose of arthritis and ulcerative colitis medicine tofacitinib (Xeljanz, Xeljanz XR). U.S. Food and Drug Administration (FDA) (Podcast). 5 August 2019. Retrieved 15 December 2019.
- "FDA approves Boxed Warning about increased risk of blood clots and death with higher dose of arthritis and ulcerative colitis medicine tofacitinib (Xeljanz, Xeljanz XR)". U.S. Food and Drug Administration. 15 December 2019. Archived from the original on 15 December 2019. Retrieved 15 December 2019.
- "FDA approves Xeljanz for rheumatoid arthritis". U.S. Food and Drug Administration (FDA) (Press release). 6 November 2012. Archived from the original on 2 April 2014.
- Pfizer Provides Update on Global Regulatory Approvals and Launches of XELJANZ® (tofacitinib citrate) for the Treatment of Rheumatoid Arthritis. July 2013
- Nordqvist C (27 April 2013). "Pfizer's Arthritis Drug Xeljanz (tofacitinib) Receives A Negative Opinion In Europe". Medical News Today. Retrieved 2 August 2013.
- McKee S (29 June 2018). "EU approves Pfizer's Xeljanz for psoriatic arthritis". PharmaTimes. Retrieved 3 June 2019.
- "XELJANZ- tofacitinib tablet, film coated / XELJANZ XR- tofacitinib tablet, film coated, extended release". DailyMed. 30 August 2019.
- FDA Warns of Risk for PE, Death With Higher Dose Tofacitinib (Xeljanz) for RA - Medscape - Feb 25, 2019.
- "Tofacitinib". Drugs in R&D. 10 (4): 271–84. 2010. doi:10.2165/11588080-000000000-00000. PMC 3585773. PMID 21171673.
- Ghoreschi K, Jesson MI, Li X, Lee JL, Ghosh S, Alsup JW, et al. (April 2011). "Modulation of innate and adaptive immune responses by tofacitinib (CP-690,550)". Journal of Immunology. 186 (7): 4234–43. doi:10.4049/jimmunol.1003668. PMC 3108067. PMID 21383241.
- "Seeking Profit for Taxpayers in Potential of New Drug", Jonathan Weisman, New York Times, 18 March 2013 (subscription firewall)
- Garber K (January 2013). "Pfizer's first-in-class JAK inhibitor pricey for rheumatoid arthritis market". Nature Biotechnology. 31 (1): 3–4. doi:10.1038/nbt0113-3. PMID 23302910.
- Kremer JM, Bloom BJ, Breedveld FC, Coombs JH, Fletcher MP, Gruben D, et al. (July 2009). "The safety and efficacy of a JAK inhibitor in patients with active rheumatoid arthritis: Results of a double-blind, placebo-controlled phase IIa trial of three dosage levels of CP-690,550 versus placebo". Arthritis and Rheumatism. 60 (7): 1895–905. doi:10.1002/art.24567. PMID 19565475.
- Herper M (2 March 2011). "Why Pfizer's Biggest Experimental Drug Got A Name Change". Forbes. Retrieved 3 March 2011.
- Moisan A, et al. (2014). "White-to-brown metabolic conversion of human adipocytes by JAK inhibition". Nature Cell Biology. 17: 57–67. doi:10.1038/ncb3075. PMC 4276482.
- Di Lernia V, Bardazzi F (1 January 2016). "Profile of tofacitinib citrate and its potential in the treatment of moderate-to-severe chronic plaque psoriasis". Drug Design, Development and Therapy. 10: 533–9. doi:10.2147/DDDT.S82599. PMC 4743637. PMID 26889081.
- Vuitton L, Koch S, Peyrin-Biroulet L (November 2013). "Janus kinase inhibition with tofacitinib: changing the face of inflammatory bowel disease treatment". Current Drug Targets. 14 (12): 1385–91. doi:10.2174/13894501113149990160. PMID 23627915.
- Zand MS (July 2013). "Tofacitinab in renal transplantation". Transplantation Reviews. 27 (3): 85–9. doi:10.1016/j.trre.2013.04.001. PMC 3713609. PMID 23849222.
- Kirk AD, Knechtle SJ, Larsen CP, Madsen JC, Pearson TC, Webber SA (21 July 2014). Textbook of Organ Transplantation Set. John Wiley & Sons. pp. 245–. ISBN 978-1-118-88962-6.
- Wojciechowski D, Vincenti F (September 2013). "Tofacitinib in kidney transplantation". Expert Opinion on Investigational Drugs. 22 (9): 1193–9. doi:10.1517/13543784.2013.811231. PMID 23841583.
- Myrvang H (June 2012). "Transplantation: Tofacitinib safe and effective in renal transplant recipients". Nature Reviews. Nephrology. 8 (8): 432. doi:10.1038/nrneph.2012.120. PMID 22735765.
- Kalluri HV, Hardinger KL (August 2012). "Current state of renal transplant immunosuppression: Present and future". World Journal of Transplantation. 2 (4): 51–68. doi:10.5500/WJT.v2.i4.51. PMC 3782235. PMID 24175197.
- Papp KA, Menter MA, Abe M, Elewski B, Feldman SR, Gottlieb AB, et al. (October 2015). "Tofacitinib, an oral Janus kinase inhibitor, for the treatment of chronic plaque psoriasis: results from two randomized, placebo-controlled, phase III trials". The British Journal of Dermatology. 173 (4): 949–61. doi:10.1111/bjd.14018. PMID 26149717.
- Bachelez H, van de Kerkhof PC, Strohal R, Kubanov A, Valenzuela F, Lee JH, et al. (August 2015). "Tofacitinib versus etanercept or placebo in moderate-to-severe chronic plaque psoriasis: a phase 3 randomised non-inferiority trial". Lancet. 386 (9993): 552–61. doi:10.1016/S0140-6736(14)62113-9. PMID 26051365.
- "Pfizer Receives Complete Response Letter from FDA for Oral XELJANZ® (tofacitinib citrate) Supplemental New Drug Application for Moderate to Severe Chronic Plaque Psoriasis" (Press release). Pfizer. 14 October 2015.
- Xing L, Dai Z, Jabbari A, Cerise JE, Higgins CA, Gong W, et al. (September 2014). "Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition". Nature Medicine. 20 (9): 1043–9. doi:10.1038/nm.3645. PMC 4362521. PMID 25129481.
- Craiglow BG, King BA (December 2014). "Killing two birds with one stone: oral tofacitinib reverses alopecia universalis in a patient with plaque psoriasis". The Journal of Investigative Dermatology. 134 (12): 2988–2990. doi:10.1038/jid.2014.260. PMID 24940651.
- Jabbari A, Nguyen N, Cerise JE, Ulerio G, de Jong A, Clynes R, et al. (August 2016). "Treatment of an alopecia areata patient with tofacitinib results in regrowth of hair and changes in serum and skin biomarkers". Experimental Dermatology. 25 (8): 642–3. doi:10.1111/exd.13060. PMC 4963264. PMID 27119625.
- Kennedy Crispin M, Ko JM, Craiglow BG, Li S, Shankar G, Urban JR, et al. (September 2016). "Safety and efficacy of the JAK inhibitor tofacitinib citrate in patients with alopecia areata". JCI Insight. 1 (15): e89776. doi:10.1172/jci.insight.89776. PMC 5033755. PMID 27699252.
- Mackay-Wiggan J, Jabbari A, Nguyen N, Cerise JE, Clark C, Ulerio G, et al. (September 2016). "Oral ruxolitinib induces hair regrowth in patients with moderate-to-severe alopecia areata". JCI Insight. 1 (15): e89790. doi:10.1172/jci.insight.89790. PMC 5033756. PMID 27699253.
- Craiglow BG, King BA (October 2015). "Tofacitinib Citrate for the Treatment of Vitiligo: A Pathogenesis-Directed Therapy". JAMA Dermatology. 151 (10): 1110–2. doi:10.1001/jamadermatol.2015.1520. PMID 26107994.
- Levy LL, Urban J, King BA (September 2015). "Treatment of recalcitrant atopic dermatitis with the oral Janus kinase inhibitor tofacitinib citrate". Journal of the American Academy of Dermatology. 73 (3): 395–9. doi:10.1016/j.jaad.2015.06.045. PMID 26194706.
- AS: Is Tofacitinib the Next Big Thing? Nov 2016
External links
- "Tofacitinib". Drug Information Portal. U.S. National Library of Medicine.