Iron–sulfur protein
Iron–sulfur proteins are proteins characterized by the presence of iron–sulfur clusters containing sulfide-linked di-, tri-, and tetrairon centers in variable oxidation states. Iron–sulfur clusters are found in a variety of metalloproteins, such as the ferredoxins, as well as NADH dehydrogenase, hydrogenases, coenzyme Q – cytochrome c reductase, succinate – coenzyme Q reductase and nitrogenase.[1] Iron–sulfur clusters are best known for their role in the oxidation-reduction reactions of electron transport in mitochondria and chloroplasts. Both Complex I and Complex II of oxidative phosphorylation have multiple Fe–S clusters. They have many other functions including catalysis as illustrated by aconitase, generation of radicals as illustrated by SAM-dependent enzymes, and as sulfur donors in the biosynthesis of lipoic acid and biotin. Additionally, some Fe–S proteins regulate gene expression. Fe–S proteins are vulnerable to attack by biogenic nitric oxide, forming dinitrosyl iron complexes. In most Fe–S proteins, the terminal ligands on Fe are thiolate, but exceptions exist.[2]
The prevalence of these proteins on the metabolic pathways of most organisms leads some scientists to theorize that iron–sulfur compounds had a significant role in the origin of life in the iron–sulfur world theory.
Structural motifs
In almost all Fe–S proteins, the Fe centers are tetrahedral and the terminal ligands are thiolato sulfur centers from cysteinyl residues. The sulfide groups are either two- or three-coordinated. Three distinct kinds of Fe–S clusters with these features are most common.
2Fe–2S clusters
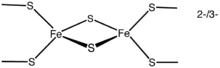
The simplest polymetallic system, the [Fe2S2] cluster, is constituted by two iron ions bridged by two sulfide ions and coordinated by four cysteinyl ligands (in Fe2S2 ferredoxins) or by two cysteines and two histidines (in Rieske proteins). The oxidized proteins contain two Fe3+ ions, whereas the reduced proteins contain one Fe3+ and one Fe2+ ion. These species exist in two oxidation states, (FeIII)2 and FeIIIFeII. CDGSH iron sulfur domain is also associated with 2Fe-2S clusters.
4Fe–4S clusters
A common motif features a four iron ions and four sulfide ions placed at the vertices of a cubane-type cluster. The Fe centers are typically further coordinated by cysteinyl ligands. The [Fe4S4] electron-transfer proteins ([Fe4S4] ferredoxins) may be further subdivided into low-potential (bacterial-type) and high-potential (HiPIP) ferredoxins. Low- and high-potential ferredoxins are related by the following redox scheme:
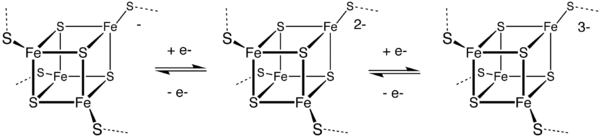
In HiPIP, the cluster shuttles between [2Fe3+, 2Fe2+] (Fe4S42+) and [3Fe3+, Fe2+] (Fe4S43+). The potentials for this redox couple range from 0.4 to 0.1 V. In the bacterial ferredoxins, the pair of oxidation states are [Fe3+, 3Fe2+] (Fe4S4+) and [2Fe3+, 2Fe2+] (Fe4S42+). The potentials for this redox couple range from −0.3 to −0.7 V. The two families of 4Fe–4S clusters share the Fe4S42+ oxidation state. The difference in the redox couples is attributed to the degree of hydrogen bonding, which strongly modifies the basicity of the cysteinyl thiolate ligands. A further redox couple, which is still more reducing than the bacterial ferredoxins is implicated in the nitrogenase.
Some 4Fe–4S clusters bind substrates and are thus classified as enzyme cofactors. In aconitase, the Fe–S cluster binds aconitate at the one Fe centre that lacks a thiolate ligand. The cluster does not undergo redox, but serves as a Lewis acid catalyst to convert citrate to isocitrate. In radical SAM enzymes, the cluster binds and reduces S-adenosylmethionine to generate a radical, which is involved in many biosyntheses.[3]
3Fe–4S clusters
Proteins are also known to contain [Fe3S4] centres, which feature one iron less than the more common [Fe4S4] cores. Three sulfide ions bridge two iron ions each, while the fourth sulfide bridges three iron ions. Their formal oxidation states may vary from [Fe3S4]+ (all-Fe3+ form) to [Fe3S4]2− (all-Fe2+ form). In a number of iron–sulfur proteins, the [Fe4S4] cluster can be reversibly converted by oxidation and loss of one iron ion to a [Fe3S4] cluster. E.g., the inactive form of aconitase possesses an [Fe3S4] and is activated by addition of Fe2+ and reductant.
Other Fe–S clusters
More complex polymetallic systems are common. Examples include both the 8Fe and the 7Fe clusters in nitrogenase. Carbon monoxide dehydrogenase and the [FeFe]-hydrogenase also feature unusual Fe–S clusters. A special 6 cysteine-coordinated [Fe4S3] cluster was found in oxygen-tolerant membrane-bound [NiFe] hydrogenases.[4][5]
Biosynthesis
The biosynthesis of the Fe–S clusters has been well studied.[6][7][8] The biogenesis of iron sulfur clusters has been studied most extensively in the bacteria E. coli and A. vinelandii and yeast S. cerevisiae. At least three different biosynthetic systems have been identified so far, namely nif, suf, and isc systems, which were first identified in bacteria. The nif system is responsible for the clusters in the enzyme nitrogenase. The suf and isc systems are more general.
The yeast isc system is the best described. Several proteins constitute the biosynthetic machinery via the isc pathway. The process occurs in two major steps: (1) the Fe/S cluster is assembled on a scaffold protein followed by (2) transfer of the preformed cluster to the recipient proteins. The first step of this process occurs in the cytoplasm of prokaryotic organisms or in the mitochondria of eukaryotic organisms. In the higher organisms the clusters are therefore transported out of the mitochondrion to be incorporated into the extramitochondrial enzymes. These organisms also possess a set of proteins involved in the Fe/S clusters transport and incorporation processes that are not homologous to proteins found in prokaryotic systems.
Synthetic analogues
Synthetic analogues of the naturally occurring Fe–S clusters were first reported by Holm and coworkers.[9] Treatment of iron salts with a mixture of thiolates and sulfide affords derivatives such as (Et4N)2Fe4S4(SCH2Ph)4].[10][11]
References
- S. J. Lippard, J. M. Berg “Principles of Bioinorganic Chemistry” University Science Books: Mill Valley, CA; 1994. ISBN 0-935702-73-3.
- Bak, D. W.; Elliott, S. J. (2014). "Alternative FeS cluster ligands: tuning redox potentials and chemistry". Curr. Opin. Chem. Biol. 19: 50–58. doi:10.1016/j.cbpa.2013.12.015.
- Susan C. Wang; Perry A. Frey (2007). "S-adenosylmethionine as an oxidant: the radical SAM superfamily". Trends in Biochemical Sciences. 32 (3): 101–10. doi:10.1016/j.tibs.2007.01.002. PMID 17291766.
- Fritsch, J; Scheerer, P; Frielingsdorf, S; Kroschinsky, S; Friedrich, B; Lenz, O; Spahn, CMT (2011-10-16). "The crystal structure of an oxygen-tolerant hydrogenase uncovers a novel iron-sulphur centre". Nature. 479 (7372): 249–252. doi:10.1038/nature10505. PMID 22002606.
- Shomura, Y; Yoon, KS; Nishihara, H; Higuchi, Y (2011-10-16). "Structural basis for a [4Fe-3S] cluster in the oxygen-tolerant membrane-bound [NiFe]-hydrogenase". Nature. 479 (7372): 253–256. doi:10.1038/nature10504. PMID 22002607.
- Johnson D, Dean DR, Smith AD, Johnson MK (2005). "Structure, function and formation of biological iron–sulfur clusters". Annual Review of Biochemistry. 74 (1): 247–281. doi:10.1146/annurev.biochem.74.082803.133518. PMID 15952888.
- Johnson, M.K. and Smith, A.D. (2005) Iron–sulfur proteins in: Encyclopedia of Inorganic Chemistry (King, R.B., Ed.), 2nd edn, John Wiley & Sons, Chichester.
- Lill R, Mühlenhoff U (2005). "Iron–sulfur-protein biogenesis in eukaryotes". Trends in Biochemical Sciences. 30 (3): 133–141. doi:10.1016/j.tibs.2005.01.006. PMID 15752985.
- T. Herskovitz; B. A. Averill; R. H. Holm; J. A. Ibers; W. D. Phillips; J. F. Weiher (1972). "Structure and Properties of a Synthetic Analogue of Bacterial Iron-Sulfur Proteins". Proceedings of the National Academy of Sciences. 69 (9): 2437–2441. doi:10.1073/pnas.69.9.2437. PMC 426959. PMID 4506765.
- Holm, R. H.; Lo, W. (2016). "Structural Conversions of Synthetic and Protein-Bound Iron-Sulfur Clusters". Chem. Rev. 116: 13685–13713. doi:10.1021/acs.chemrev.6b00276.
- Lee, S. C.; Lo, W.; Holm, R. H. (2014). "Developments in the Biomimetic Chemistry of Cubane-Type and Higher Nuclearity Iron–Sulfur Clusters". Chemical Reviews. 114: 3579–3600. doi:10.1021/cr4004067. PMC 3982595. PMID 24410527.
Further reading
- Beinert, H. (2000). "Iron-sulfur proteins: ancient structures, still full of surprises". J. Biol. Inorg. Chem. 5 (1): 2–15. doi:10.1007/s007750050002. PMID 10766431.
- Beinert, H.; Kiley, P.J. (1999). "Fe-S proteins in sensing and regulatory functions". Curr. Opin. Chem. Biol. 3 (2): 152–157. doi:10.1016/S1367-5931(99)80027-1. PMID 10226040.
- Johnson, M.K. (1998). "Iron-sulfur proteins: new roles for old clusters". Curr. Opin. Chem. Biol. 2 (2): 173–181. doi:10.1016/S1367-5931(98)80058-6. PMID 9667933.
- Nomenclature Committee of the International Union of Biochemistry (NC-IUB) (1979). "Nomenclature of iron-sulfur proteins. Recommendations 1978". Eur. J. Biochem. 93 (3): 427–430. doi:10.1111/j.1432-1033.1979.tb12839.x. PMID 421685.
- Noodleman, L., Lovell, T., Liu, T., Himo, F. and Torres, R.A. (2002). "Insights into properties and energetics of iron-sulfur proteins from simple clusters to nitrogenase". Curr. Opin. Chem. Biol. 6 (2): 259–273. doi:10.1016/S1367-5931(02)00309-5. PMID 12039013.CS1 maint: multiple names: authors list (link)
- Spiro, T.G., Ed. (1982). Iron-sulfur proteins. New York: Wiley. ISBN 0-471-07738-0.CS1 maint: multiple names: authors list (link)
External links
- Iron-Sulfur+Proteins at the US National Library of Medicine Medical Subject Headings (MeSH)
- Examples of iron-sulfur clusters