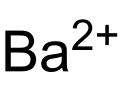Barium ferrate
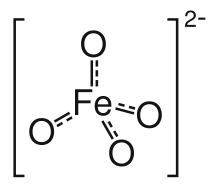
Barium ferrate is the chemical compound of formula BaFeO4. This is a rare compound containing iron in the +6 oxidation state.[2] The ferrate(VI) ion has two unpaired electrons, making it paramagnetic.[3] It is isostructural with BaSO4, and contains the tetrahedral [FeO4]2− anion.[4]
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Barium ferrate(VI) | |||
| Other names
Barium ferrate(2-) | |||
| Identifiers | |||
3D model (JSmol) |
|||
| |||
| |||
| Properties | |||
| BaFeO4 | |||
| Molar mass | 257,1646 g/mol | ||
| Appearance | Dark red, opaque crystals | ||
| insoluble | |||
| Structure | |||
| orthorhombic | |||
| Pnma, No. 62[1] | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Structure
The ferrate(VI) anion is paramagnetic due to its two unpaired electrons and it has a tetrahedral molecular geometry.[3]
X-ray diffraction has been used to determine the orthorhombic unit cell structure[1] (lattice vectors a ≠ b ≠ c, interaxial angles α=β=γ=90°)[5] of nanocrystalline BaFeO4. It crystallized in the Pnma space group (point group: D2h) with lattice parameters a=0.8880 nm, b=0.5512 nm and c=0.7214 nm.[1] The accuracy of the X-Ray diffraction data has been verified by the lattice fringe intervals from High-Resolution Transmission Electron Microscopy (HRTEM) and cell parameters calculated from Selected Area Diffraction (SAED).[1]

Characterization
Infrared absorbance peaks of barium ferrate are observed at 870, 812, 780 cm−1.[7]

BaFeO4 has a magnetic moment of (3.20 ± 0.09) x Am2 (3.45 ± 0.1BM) with a Weiss constant of -89 K.[9]
Preparation and Chemistry
There are two methods for producing ferrate (VI): dry and wet synthetic methods.
The dry synthetic method is usually performed using a thermal technique.[7] The wet method employs chemical and electrochemical techniques. Addition of a soluble barium salt to an alkali metal ferrate solution produces a maroon precipitate of barium ferrate, a crystal which has the same structure as barium chromate and has approximately the same solubility.[10] Barium ferrate, BaFeO4, can be prepared by adding barium oxide to a mixture NaClO and ferric nitrate at room temperature (or 0 °C).[11] Primary experiments indicate an improvement in the purity of the synthesized barium ferrate by performing the reaction at low temperature in the absence of carbon dioxide and by rapidly filtering and drying the precipitate.[10]
Uses
Barium ferrate is an oxidizing agent and is used as an oxidizing reagent in organic syntheses. Its other applications include removal of color, removal of cyanide, killing bacteria and contaminated and waste water treatment.[7]
Salts of ferrate (VI) are energetic cathode materials in “super-iron” batteries. Cathodes containing ferrate (VI) compounds are referred to as “super-iron” cathodes due to their highly oxidized iron basis, multiple electron transfer, and high intrinsic energy. Among all ferrate (VI) salts, barium ferrate sustains unusually facile charge transfer, which is important for the high power domain of alkaline batteries.[8]
Reactions
Barium ferrate is the most stable of the ferrate(VI) compounds. It can be prepared in its purest state and has the most definite composition. Barium ferrate can be easily decomposed by all soluble acids, including carbonic acid. If carbon dioxide is passed through water on which hydrated barium ferrate is suspended, barium ferrate will decompose completely to form barium carbonate, ferric hydroxide and oxygen gas. Alkaline sulfates decompose barium ferrate that has not been dried, forming barium sulfate, ferric hydroxide and oxygen gas.
See also
References
- Ni, Xiao-Min; Ji, Ming-Rong; Yang, Zhi-Ping; Zheng, Hua-Gui (15 January 2004). "Preparation and structure characterization of nanocrystalline BaFeO4". Journal of Crystal Growth. 261 (1): 82–86. doi:10.1016/j.jcrysgro.2003.09.024.
- Briggs, J. G. R. (2005). Longman A-level course in chemistry (4th ed.). Pearson Education South Asia. p. 536. ISBN 978-981-4105-08-8.
- Wiberg, Egon; Wiberg, Nils; Holleman, Arnold (2001). Inorganic chemistry. Academic Press. pp. 1457–1458. ISBN 978-0-12-352651-9.
- Wells, A.F. (1986). Structural inorganic chemistry (5th ed.). Oxford [Oxfordshire]: Clarendon Press. ISBN 978-0-19-855370-0.
- "IUCr". www.iucr.org. Retrieved 2016-04-29.
- Ropp, Richard C. (2012-12-31). Encyclopedia of the Alkaline Earth Compounds. Newnes. ISBN 9780444595539.
- Henry-Chase, Adonica; Tewari, Brij Bhushan (20 September 2013). "Use to Ferrate (VI) A Green Chemical for the Environment Remediation" (PDF). Revista Boliviana de Química. 30 (1): 13–23. ISSN 0250-5460. Retrieved 29 April 2016.
- Licht, Stuart; Naschitz, Vera; Wang, Baohui (2002). "Rapid Chemical Synthesis of the barium ferrate super-iron Fe (VI) compound, BaFeO4". Journal of Power Sources. 109: 67–70. doi:10.1016/s0378-7753(02)00041-1.
- Audette, R. J.; Quail, J. W. (1972). "Potassium, rubidium, cesium, and barium ferrates(VI). Preparations, infrared spectra, and magnetic susceptibilities". Inorganic Chemistry. 11 (8): 1904–1908. doi:10.1021/ic50114a034.
- Gump, J. R.; Wagner, W. F.; schreyer, J. M. (1954-12-01). "Preparation and Analysis of Barium Ferrate(VI)". Analytical Chemistry. 26 (12): 1957. doi:10.1021/ac60096a027. ISSN 0003-2700.
- Herber, Rolfe H.; Johnson, David (1979). "Lattice dynamics and hyperfine interactions in M2FeO4 (M = potassium(1+), rubidium(1+), cesium(1+)) and M'FeO4 (M' = strontium(2+), barium(2+))". Inorganic Chemistry. 18 (10): 2786–2790. doi:10.1021/ic50200a030.